|
 |
| J Rhinol > Volume 31(1); 2024 |
|
Abstract
Background and Objectives
Methods
Results
Conclusion
Supplementary Materials
Supplementary Table 1.
Notes
Availability of Data and Material
All data generated or analyzed during the study are included in this published article and its supplementary information files.
Conflicts of Interest
Do Hyun Kim and Se Hwan Hwang who are on the editorial board of the Journal of Rhinology were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: all authors. Data curation: Sun Hong Kim, Do Hyun Kim, Se Hwan Hwang. Formal analysis: Ah Young Bae, Do Hyun Kim, Se Hwan Hwang. Funding acquisition: Do Hyun Kim, Se Hwan Hwang. Investigation: all authors. Methodology: all authors. Project administration: Do Hyun Kim, Se Hwan Hwang. Resources: all authors. Software: Do Hyun Kim, Se Hwan Hwang. Supervision: Do Hyun Kim, Se Hwan Hwang. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1066232) and the Institute of Clinical Medicine Research of Bucheon St. Mary’s Hospital, Research Fund (2022). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Fig. 2.
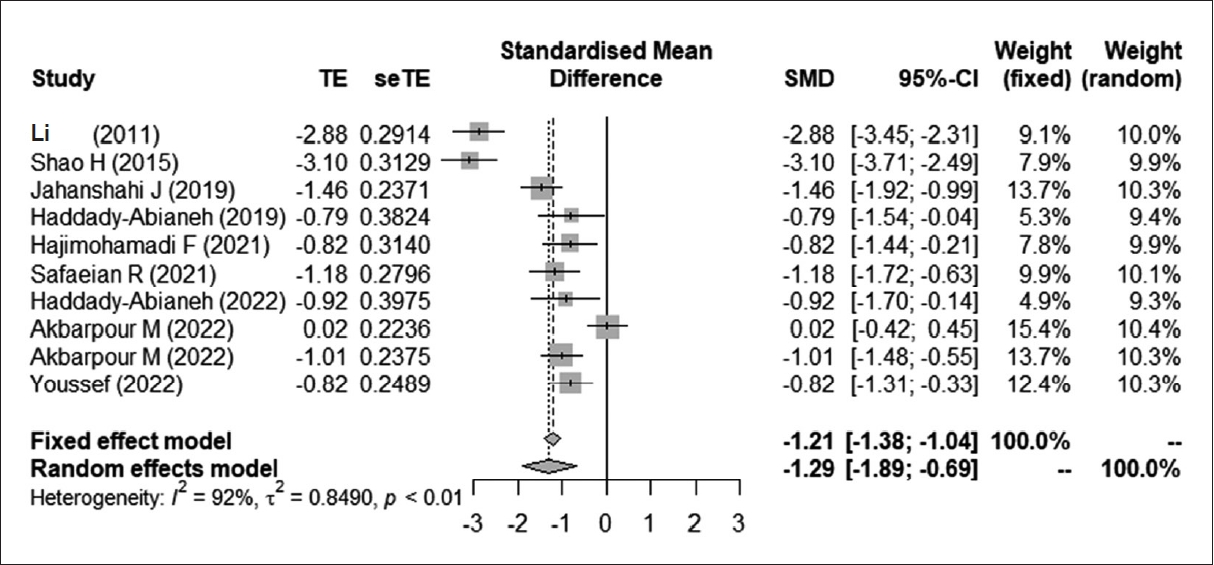
Fig. 3.
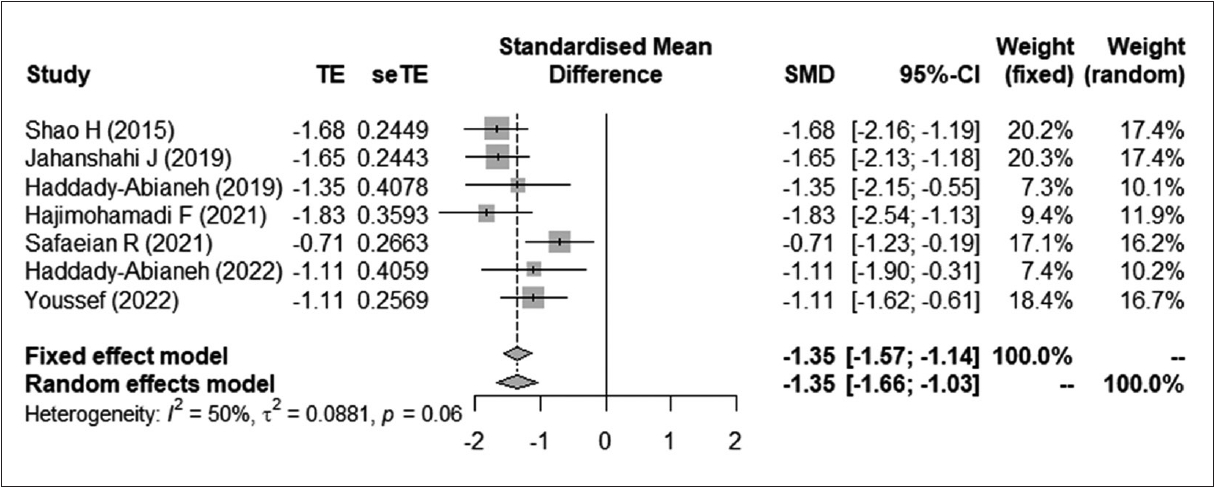
Fig. 4.

Fig. 5.

Fig. 6.
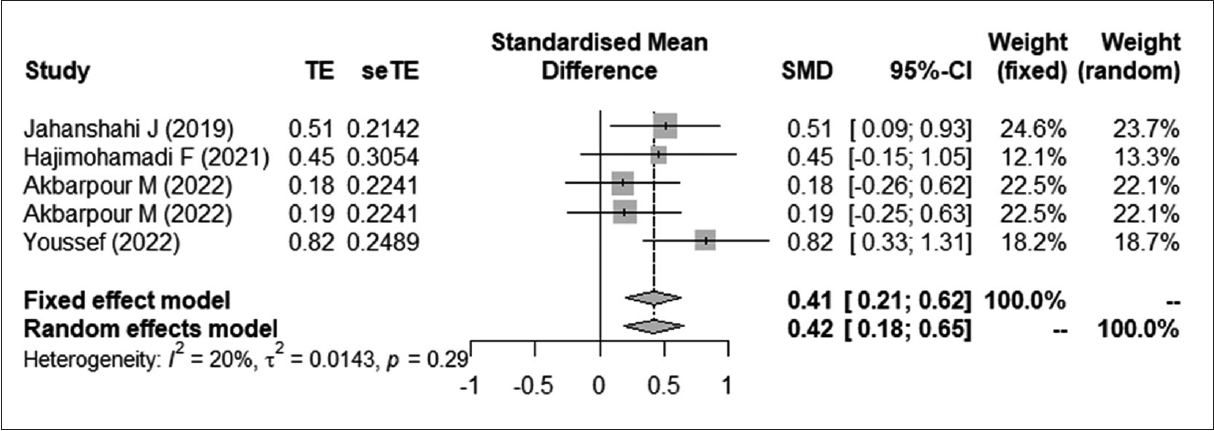
Fig. 7.

Table 1.
| Study | Year | Surgery | Age (years; mean, range, or standard deviation) | Sex (male/female) | Nation | Route of administration | Medication | Comparison | Measures |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. [16] | 2011 | ESS | NR | NR | China | Intravenous | 0.15–0.3 µg/kg diluted in 100 mL of normal saline before surgery | 100 mL of normal saline, administered intravenously | Intraoperative blood loss, operation time |
| Shao et al. [7] | 2015 | ESS | 18–65 (45) | 50/40 | China | Intravenous | 0.3 µg/kg preoperatively | Normal saline | Surgical blood loss (mL), quality of surgical field (10), duration of surgery (min) |
| Jahanshahi et al. [8] | 2019 | ESS | 18–60 (40.27) | 51/39 | Iran | Nasal | Preoperative single puff (10 µg) of nasal desmopressin in each side of the nasal cavity (20 µg total), 30 min before surgery | Normal saline spray | Mean arterial pressure (mm Hg), duration of surgery, bleeding volume (total), bleeding score (Boezaart scale), quality of surgical field, sodium level of serum after surgery |
| Haddady-Abianeh et al. [9] | 2019 | Open rhinoplasty | NR | NR | Iran | Nasal | Nasal spray of desmopressin 60 min before surgery | Saline spray | Operative bleeding volume (cc), bleeding score (Boezaart scale), the Likert score (the surgeon’s satisfaction), duration of open septorhinoplasty (min) |
| Hajimohamadi et al. [13] | 2021 | ESS | 15–60 (40.14) | 31/13 | Iran | Intravenous | Maximum dose of 0.2 µg/kg | None | Duration of surgery (min), postoperative sodium serum level (mEq/L), maximum systolic blood pressure, bleeding amount, bleeding score (Boezaart scale), concentration of sodium between 8 and 12 h after surgery |
| Safaeian et al. [14] | 2021 | ESS | 18–66 (39) | 45/15 | Iran | Nasal | Desmex nasal spray | None | Duration of surgery (min), bleeding score (Boezaart scale), bleeding amount (cc), surgeon satisfied with the surgical field |
| Haddady-Abianeh et al. [11] | 2022 | Open rhinoplasty | 30.67±8.27 | NR | Iran | Nasal | Dose of 40 µg (2 puffs of 0.1 mg/mL spray in each nostril) | Normal saline spray | Operative bleeding, Boezaart score (quality of the surgical field), Likert score (surgeon’s satisfaction) |
| Akbarpour et al. [15] | 2022 | ESS | 18–60 (41.0) | 80/40 | Iran | Nasal | 20 µg, a single dose of Desmex nasal spray, 10 g/dose+a single dose of NaCl 0.65% nasal spray | Double dose of NaCl 0.65% nasal spray | Sodium serum level, blood pressure, duration of surgery, blood loss |
| Youssefy et al. [10] | 2022 | Open rhinoplasty | 30.67±8.27 | 33/37 | Iran | Intravenous | 0.1–0.2 µg/kg infused in 500 cc of normal saline serum, 30 min prior to surgery | 500 mL of normal saline | Operation time (min), sodium serum level (mEq/L), mean arterial blood pressure, blood loss (cc), bleeding score (Boezaart scale) |





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print