|
 |
| J Rhinol > Volume 30(3); 2023 |
|
Abstract
Background and Objectives
Methods
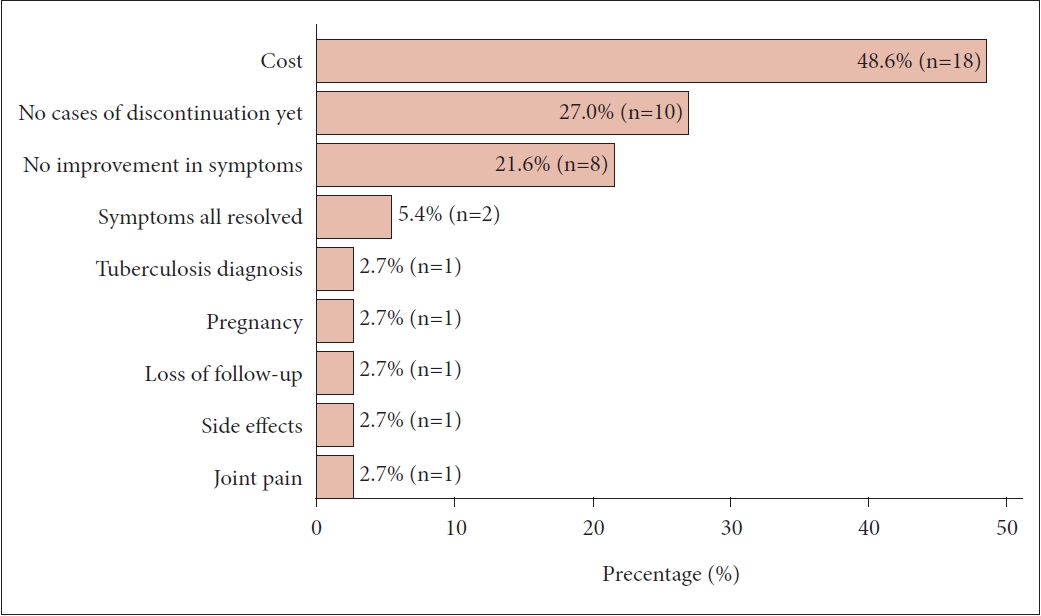
Results
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Gwanghui Ryu who is on the editorial board of the Journal of Rhinology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: all authors. Data curation: Gwanghui Ryu, Shin Hyuk Yoo. Formal analysis: Hyunkyung Cha, Gwanghui Ryu. Investigation: all authors. Methodology: Gwanghui Ryu, Shin Hyuk Yoo, Ji-Hun Mo. Visualization: Hyunkyung Cha. Writing—original draft: Hyunkyung Cha, Gwanghui Ryu. Writing—review & editing: Shin Hyuk Yoo, Ji-Hun Mo.
Fig. 1.
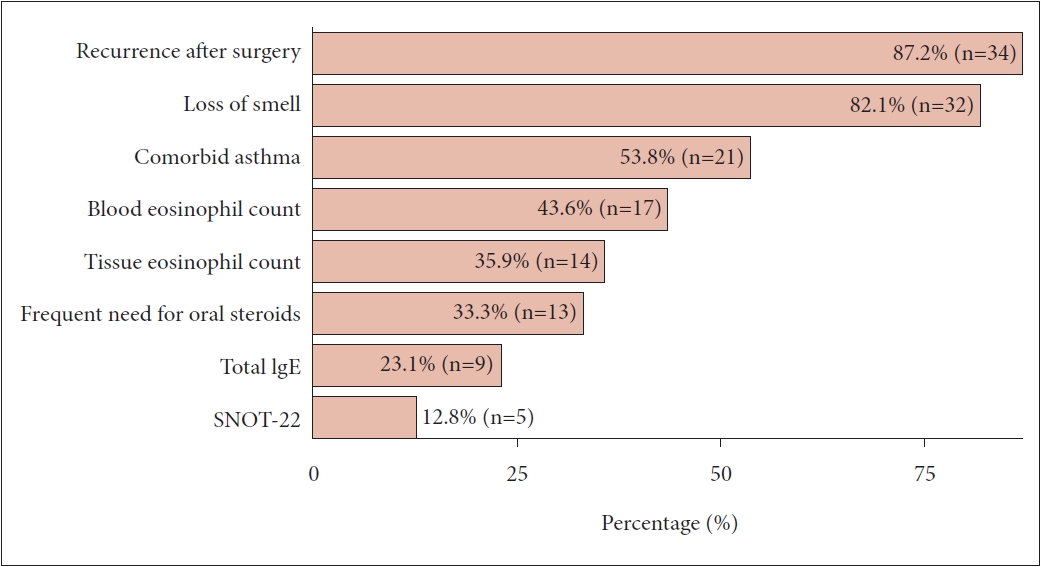
Table 1.
Table 2.
Table 3.
Table 4.
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,426 View
- 41 Download
- Related articles
-
Practical Review of Biologics in Chronic Rhinosinusitis With Nasal Polyps2021 November;28(3)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print