|
 |
| J Rhinol > Volume 31(1); 2024 |
|
Abstract
Background and Objectives
Sinonasal fungal balls (FBs) most commonly occur in the maxillary sinus, followed by the sphenoid sinus (SS). Relatively little is known about the predisposing factors and pathogenesis of unilateral sphenoid sinus fungal balls (SSFBs) compared to maxillary sinus FBs. We investigated whether anatomical variations have clinical implications for the location of unilateral SSFBs.
Methods
This study included 33 patients who underwent endoscopic sinus surgery for unilateral SSFBs between 2010 and 2021. Preoperative computed tomography scans were used to analyze the presence of anatomical variations, including sphenoid lateral recess, complete accessory septum of the SS, types of SS pneumatization, anterior and posterior nasal septal deviation (NSD), cephalocaudal NSD, concha bullosa (CB), Haller cell (HC), paradoxical middle turbinate (MT), everted uncinated process (UP), and Onodi cell.
Results
The presence of HC (33.3% vs. 12.1%, p=0.04), complete accessory septum of the SS (51.6% vs. 25.8%, p=0.04), and the sellar type of the SS (90.9% vs. 50%, p=0.003) differed significantly according to the presence or absence of FBs in the SS. However, other anatomical variations, including NSD, CB, paradoxical MT, everted UP, Onodi cell, and sphenoid lateral recess, were not significantly associated with the presence of unilateral SSFBs (all p>0.05). In the multivariable analysis, only sellar-type pneumatization of the SS showed a statistically significant relationship with SSFB, not the combined conchal and presellar type (adjusted odds ratio, 8.96; 95% confidence interval, 1.27–63.19; p=0.03).
Sinonasal fungal balls (FBs) are non-invasive fungal infections that typically affect the sinuses of immunocompetent patients [1]. The most common causative organism is Aspergillus sp., which is found most frequently in elderly women [2]. FBs predominantly arise in the maxillary sinus, with the sphenoid sinus (SS) being the second most common site, and they are more commonly unilateral than bilateral [3].
Numerous studies have aimed to elucidate the pathogenesis of sinonasal FBs. Since sinonasal FBs are most frequently found in the maxillary sinus, the bulk of research has concentrated on patients with maxillary sinus fungal balls (MSFBs). In contrast, sphenoid sinus fungal balls (SSFBs) have been the subject of far fewer studies. Although the pathogenesis of FBs is not completely understood, it is generally categorized into odontogenic, aerogenic, and mixed (both odontogenic and aerogenic) pathways [4]. Odontogenic risk factors include the use of dental filling materials and a history of dental procedures [5,6]. Factors such as nasal septal deviation (NSD), maxillary sinus volume, and anatomical variations in the osteomeatal unit (OMU) are considered to be aerogenic or local factors [7-9]. Our recent findings indicate a significant correlation between unilateral MSFB and ipsilateral odontogenic factors, as well as anatomical variations near the OMU. These variations include a posterior NSD towards the unaffected side and the absence of concha bullosa (CB) and infraorbital cells (Haller cells [HCs]) [7]. Regarding SSFBs, we propose that the aerogenic pathway is more likely than the odontogenic pathway to contribute to FB formation in the SS, given its anatomical position. The SS is aerated through the ostium of the sphenoethmoidal recess, and NSD may affect this aeration process [10]. Studies have suggested a link between NSD and both asymmetry and pneumatization of the SS [11]. However, NSD and other anatomical variants potentially related to the aerogenic pathway have not been thoroughly investigated in the context of unilateral SSFB formation.
Therefore, the aim of this study was to identify predisposing factors in patients with unilateral SSFBs. We categorized these factors into two groups: 1) those affecting nasal airflow and 2) those related to the SS and its surrounding structures. This categorization allowed us to concentrate on either aerogenic or local factors. To determine the factors that influence the location of unilateral SSFBs, we compared the anatomical differences between the sides of the nasal cavity and paranasal sinuses with and without FB presence.
We retrospectively analyzed the electronic medical records and preoperative computed tomography (CT) scans of patients diagnosed with unilateral SSFBs who underwent endoscopic sinus surgery at the Kyung Hee Medical Center from January 2010 to October 2021, with confirmed pathology. This study was approved by the Institutional Review Board of Kyung Hee University Hospital (IRB No. KHU 2021-12-001). Informed consent was waived due to the retrospective nature of the study. Among the 198 patients with FBs, patients with bilateral (n=2) or multiple FBs (n=163) in the paranasal sinus were excluded. Finally, we identified 33 patients with unilateral SSFBs, reviewed their demographics, and analyzed their CT scans. We compared the SSs with and without FBs within the same patients as the case and control groups, respectively, to identify anatomical differences in unilateral SSFB formation.
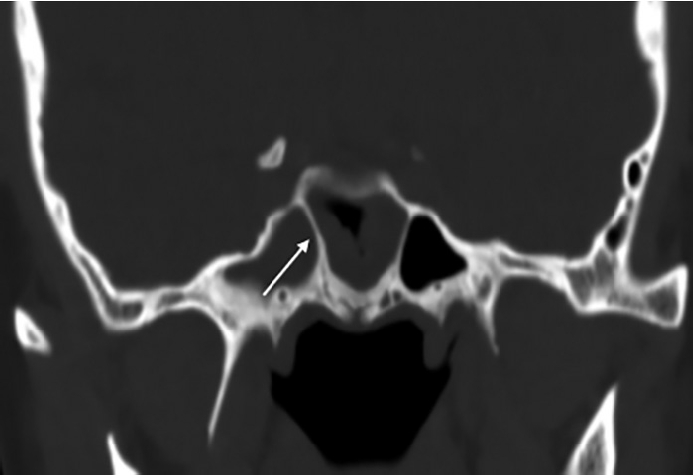
First, the effect of NSD on nasal airflow was analyzed by dividing it into anteroposterior (coronal) and cephalocaudal (axial) NSD. Anteroposterior NSD was measured at two points according to Hwang [8]: anterior (at the anterior end of the inferior turbinate; Fig. 1A) and posterior (at the ostiomeatal unit level; Fig. 1B). Furthermore, we assessed cephalocaudal NSD in the axial plane to examine the impact of nasal airflow at the natural ostium level of the SS. Cephalocaudal NSD was defined as any bending of the nasal septal contour from the straight line connecting the midpoint of the nasal tip and the sphenoidal rostrum at the level where the natural ostium of both SSs was visible (Fig. 1C). Second, the presence of CB, HC, paradoxical middle turbinate (MT), and everted uncinate process (UP) was analyzed to identify anatomical differences between the bilateral nasal passages. Third, the presence of Onodi cells, sphenoid lateral recess, complete accessory septum of the SS in the coronal plane, and the type of SS pneumatization were analyzed to evaluate the anatomical differences in the bilateral SSs and their surrounding structures. A sphenoid lateral recess is defined as a pneumatized area between the foramen rotundum and vidian canal (Fig. 2) [12]. Aksoy et al. [13] defined a complete accessory septum of the SS as the complete longitudinal division of the SS (Fig. 3). The accessory septum of the SS was defined as an additional septum rather than the main septum (dividing the left sinus from the right sinus), and the complete accessory septum was defined as the origin and insertion into the SS’ bone walls [14]. Using the main septum as a reference point, the complete accessory septum located within the left sinus was designated as the left complete accessory septum. The same method was applied to the right side as well. SS pneumatization was assessed on the sagittal view of CT scans at the level where each side’s SS was most prominently visible. It was classified into three types—conchal, presellar, and sellar—according to the relationship with the anterior wall of the sellar turcica [15]. Statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). The chi-square test was used to investigate the association between anatomical variations and the presence or absence of FB in each SS. Multivariable logistic regression analysis was performed to evaluate the predisposing factors of unilateral SSFBs. All significant predictors (p<0.05) in the univariable analyses were entered into a stepwise logistic regression analysis. Statistical significance was set at p<0.05.
The clinical characteristics of the patients with unilateral SSFBs are summarized in Table 1. The mean age of the 33 patients was 62.8 years (range, 30–86 years), of whom 13 were men and 20 were women. There were 23 cases of FBs in the left SS and 10 in the right SS. None of the patients exhibited signs of immunodeficiency. Among the various chief complaints reported by the patients, headache emerged as the predominant symptom (36%). Five patients (15%) were incidentally diagnosed. Only one patient had a history of ipsilateral dental treatment. Histopathological examination revealed 27 cases of aspergillosis and six cases of fungal disease with unspecified etiology.
Table 2 summarizes the variables identified as factors influencing the occurrence of unilateral SSFB. The prevalence of anterior NSD (48.5% vs. 48.5%) and posterior NSD (48.5% vs. 51.5%) did not show statistically significant differences according to the presence or absence of FB in the SS. Cephalocaudal NSD showed a non-statistically significant relationship with the absence of FBs in the SS (p=0.09). The presence of CB (33.3% vs. 24.2%), paradoxical MT (3.0% vs. 9.1%), everted UP (3.0% vs. 3.0%), Onodi cell (30.3% vs. 21.2%), and sphenoid lateral recess (61.3% vs. 38.7%) also did not show statistically significant differences in frequency between SSs with and without FBs (all p>0.05). Interestingly, the presence of HC (33.3% vs. 12.1%, p=0.04) and complete accessory septum of the SS (51.6% vs. 25.8%, p=0.04) were significantly different in frequency according to the presence of FB in the SS. We investigated the effect of pneumatization type on the presence of unilateral SSFBs. The prevalence of unilateral SSFBs according to pneumatization types of the SS was as follows: the pneumatization types of the SSs with FBs were conchal in 0 (0%), presellar in 2 (9.1%), and sellar in 20 (90.9%), but those of the SSs without FBs were conchal in 2 (9.1%), presellar in 9 (40.9%), and sellar in 11 (50.5%) patients. The prevalence of the conchal and presellar combined type versus the sellar type was 9.1% versus 90.9% in SSs with FBs, but 50% versus 50% in SSs without FBs (p<0.01).
In Table 3, both univariable and multivariable analyses demonstrated a significant association between anatomical factors and SSFB occurrence. HC (odds ratio [OR]=3.63; 95% confidence interval [CI], 1.02–12.93; p=0.04) and complete accessory septum of the SS (OR=3.07; 95% CI, 1.05–8.93; p=0.04) showed a statistically significant association with the occurrence of unilateral SSFBs. In other words, FBs were more likely to be present in the SS on the side with the HC or complete accessory septum of the SS. However, the presence of HC and complete accessory septum of the SS did not show a statistically significant relationship in the multivariable analysis (all p>0.05).
Regarding the association between the types of SS pneumatization and the occurrence of unilateral SSFB, the presence of the sellar type was associated with significantly higher odds of unilateral SSFB on the same side (OR=10.00; 95% CI, 1.87–53.48; p<0.01) than the conchal or presellar type (Table 3). The results of logistic regression analysis comparing pneumatization types (combined conchal and presellar versus sellar) are presented in Table 3. The presence of the sellar type was associated with significantly greater odds of unilateral SSFB (adjusted OR=8.96; 95% CI, 1.27–63.19; p=0.03) than the conchal or presellar type.
The pathogenesis of paranasal FBs remains a subject of debate, with limited understanding specifically in the context of SSFBs. The mechanisms by which these FBs develop are generally categorized as aerogenic, odontogenic, or a combination of both [4]. In cases involving MSFBs, odontogenic factors are believed to be more influential due to the proximity of the maxillary bone to the oral cavity and the potential for maxillary sinus manipulation during dental procedures. Eberhardt et al. [16] reported that the average distance from the maxillary sinus to the apex of the mesiobuccal root of the maxillary second molar is 1.97 mm. Basurrah et al. [17] found a higher incidence of tooth extractions and endodontic treatments in patients with MSFBs compared to those with normal sinuses. However, for SSFBs, the likelihood of odontogenic factors playing a role appears to be lower than for MSFBs. Consequently, our research focused on the aerogenic pathway, which involves the entry of airborne fungal spores into the sinus through the natural ostium, leading to the formation of FBs [18]. To our knowledge, this is the first study to explore the predisposing factors for the location of unilateral SSFBs as reported in the literature. The goal of this study is to identify anatomical differences between the SS with an FB and the sinus without an FB within the same individual. As such, we did not consider factors such as age, sex, underlying diseases, and immune status, which could affect the occurrence of FBs across different patients.
NSD is one of the main factors affecting nasal airflow. According to our data, the anterior and posterior NSD had no significant effect on the formation of unilateral SSFBs (Tables 2 and 3). This finding is consistent with the work of Lim et al. [19], who also reported no significant differences in NSD among patients with SSFBs. Furthermore, although NSD is common in patients with SSFBs, no statistically significant correlation has been found between the direction of the NSD and the presence of SSFBs [10]. Both of these studies evaluated NSD in the coronal plane. In our study, we observed a trend where cephalocaudal NSD toward the side without an FB was associated with the occurrence of unilateral SSFBs (20/33 cases, 60.6%) (Table 2), but this association did not reach statistical significance (p=0.09). This suggests that the concave nasal cavity resulting from NSD toward the SS side with no FB may facilitate the development of unilateral SSFB by allowing more airborne fungal spores to reach the natural ostium. Further research is necessary to clarify the precise role of NSD in the development of SSFBs.
Regarding other anatomical factors, we found that the presence of HCs was associated with an increased OR of unilateral SSFB (OR=3.63; 95% CI, 1.02–12.93; p=0.04). The reason why the presence of HC increased the likelihood of SSFB on the same side was not clear. In our previous study, the presence of HC decreased the likelihood of ipsilateral MSFB (adjusted OR=0.33, p<0.05) [7]. Some studies have reported that HCs reduce infundibular width [20]. Considering this, one possible hypothesis is that HCs may facilitate the flow of air containing fungal spores directly into the ostium of the SS on the same side, rather than into the maxillary sinus. Other anatomical factors including CB, paradoxical MT, everted UP, and Onodi cell did not exhibit significant associations with the occurrence of unilateral SSFB (Tables 2 and 3). Although relatively few studies on SSFBs have been published so far, Lim et al. [19] reported similar findings that CB was not related to the location of the SSFB. In that study, CB was found in 26.2% (116/442) of patients with unilateral FBs, but the presence of CB was not related to the location of the MSFB or SSFB.
Next, to examine the anatomical differences between the SSs on both sides, the type of pneumatization and the presence of a sphenoid lateral recess and inner septum within the sphenoid were investigated. The sellar type of the SS was identified as a significant predisposing factor to the presence of unilateral SSFBs in both univariable (OR=10.00; 95% CI, 1.87–53.48; p=0.003), and multivariable analyses (aOR=8.96; 95% CI, 1.27–63.19; p=0.03) (Table 3). Başer et al. [21] reported an association between SS volume and pneumatization type with pathologies (fungi and polyps). Their conclusion indicated the absence of a statistically significant difference in pneumatization types between the groups of polyps and fungi. However, the SS volume showed significant results. Isolated fungal chronic sphenoid sinusitis showed an association with a larger SS volume, whereas isolated sphenoid sinusitis with polyps was associated with a smaller SS volume. Considering the results of our study and that of Başer et al. [21], the larger volume of the sellar type compared to the conchal and presellar types might be related to FB formation.
The sphenoid lateral recess is a common site for spontaneous cerebrospinal fluid leaks, encephaloceles, and meningoencephaloceles [12,22]. As previously stated, we hypothesized that the sellar type of pneumatization, with a larger SS volume than the conchal or presellar type, was related to the occurrence of unilateral SSFB. Therefore, we postulated that the presence of a lateral recess in the SS, which is associated with a larger volume, might play a role in the formation of FBs. The frequency of the presence of a sphenoid lateral recess was higher in SSs with FBs than in SSs without FBs (61.3% vs. 38.7%, respectively), but the difference was not statistically significant (p>0.05).
Lastly, we speculated that the presence of a complete accessory septum in the SS could impact ventilation and potentially serve as a predisposing factor for FB formation. The presence of a complete accessory septum of the SS increased the likelihood of unilateral SSFB (OR=3.07; 95% CI, 1.05–8.93; p=0.04), but this association was not statistically significant in multivariate logistic regression analysis (Table 3).
The SS is surrounded by crucial neurovascular structures, and bone lysis without tissue invasion by fungal growth has been documented [23]. In fact, a study indicated that orbital complications occur in up to 17% of cases of SSFBs [24]. Therefore, early diagnosis and treatment of SSFB are crucial. However, SSFB is often underdiagnosed because the symptoms are nonspecific. For a better understanding of pathogenesis of unilateral SSFBs, this study aimed to identify predisposing factors influencing the location of unilateral SSFBs within individual patients. The limitations of this study involve its retrospective, single-center design, as well as the fact that it included relatively few patients (n=33) since unilateral SSFB is not a common disease.
In conclusion, we found a significant association between unilateral SSFBs and anatomical variations on the SS side where the FB was present. These variations included the pneumatization type of the SS (sellar), the presence of complete accessory septum of the SS, and HC. However, there was no statistically significant association between NSD and the presence of unilateral SSFBs. Thus, intranasal anatomical variations may play a significant role in the location of unilateral SSFBs.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Jeon Gang Doo. Data curation: all authors. Formal analysis: Hye Kyu Min. Investigation: Jeon Gang Doo, Hye Kyu Min. Methodology: Jeon Gang Doo, Jin-Young Min. Software: Jeon Gang Doo. Supervision: Jin-Young Min. Visualization: Jeon Gang Doo. Writing—original draft: Jeon Gang Doo. Writing—review & editing: all authors.
Fig. 1.
Anteroposterior (A and B) and cephalocaudal (C) nasal septal deviations. A: Anterior deviation of the nasal septum toward the left at the level of the nasal valve. B: Posterior deviation of the nasal septum toward the left at the level of the ostiomeatal unit. C: Cephalocaudal deviation of the nasal septum toward the right at the level of the both sphenoid ostia. point c, crista galli; point n, anterior nasal spine; point M, midpoint of nasal tip; point R, sphenoidal rostrum.
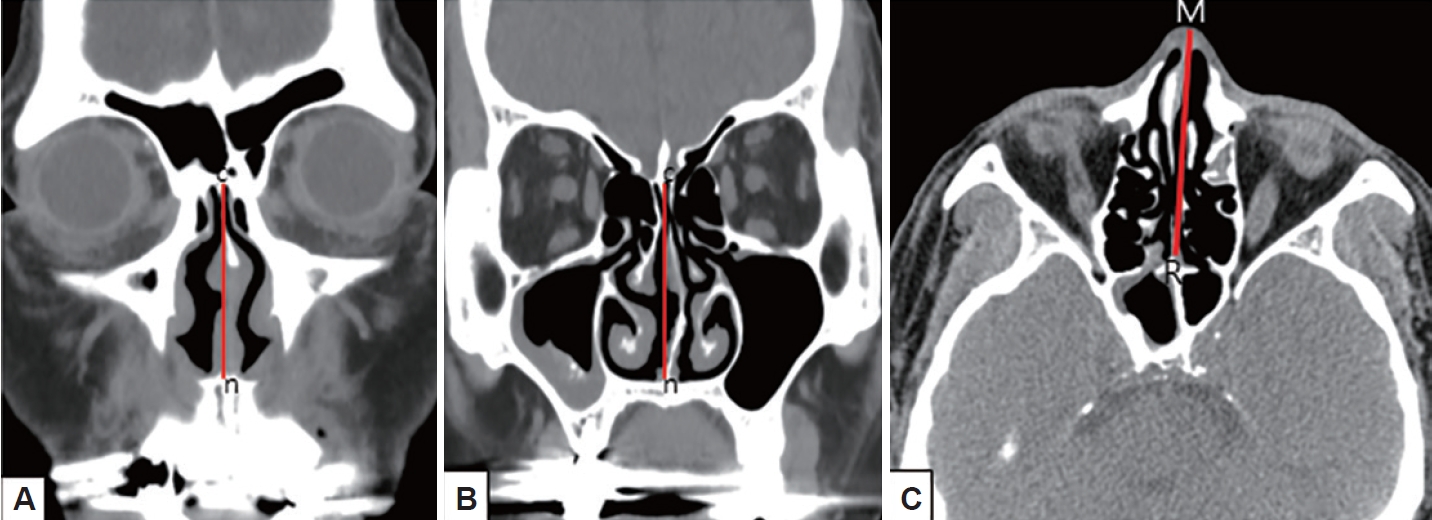
Table 1.
Clinical characteristics of patients with unilateral SSFB
Table 2.
Predisposing factors associated with unilateral sphenoid sinus fungal balls
| Variable | SS with FB (n=33) | SS without FB (n=33) | p |
|---|---|---|---|
| Nasal airflow | |||
| Anterior NSD | 16 (48.5) | 16 (48.5) | >0.99 |
| Posterior NSD | 16 (48.5) | 17 (51.5) | 0.81 |
| Cephalocaudal NSD | 13 (39.4) | 20 (60.6) | 0.09 |
| Concha bullosa | 11 (33.3) | 8 (24.2) | 0.42 |
| Haller cell | 11 (33.3) | 4 (12.1) | 0.04* |
| Paradoxical MT | 1 (3.0) | 3 (9.1) | 0.30 |
| Everted UP | 1 (3.0) | 1 (3.0) | >0.99 |
| Sphenoid sinus and surrounding structures | |||
| Onodi cell | 10 (30.3) | 7 (21.2) | 0.40 |
| Sphenoid lateral recess | 19 (61.3) | 12 (38.7) | 0.08 |
| Complete accessory septum of SS | 16 (51.6) | 8 (25.8) | 0.04* |
| Pneumatization type | Conchal+presellar 2 (9.1) | Conchal+presellar 11 (50) | <0.01** |
| Sellar 20 (90.9) | Sellar 11 (50) |
Table 3.
Associations of the predisposing factors and unilateral sphenoid fungal ball
| Variables |
Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Adjusted odds ratio (95% CI) | p | |
| Nasal airflow | ||||
| Anterior NSD | 1.00 (0.38, 2.66) | >0.99 | NA | |
| Posterior NSD | 0.89 (0.34, 2.33) | 0.81 | NA | |
| Cephalocaudal NSD | 0.42 (0.16, 1.14) | 0.09 | NA | |
| Concha bullosa | 1.56 (0.53, 4.58) | 0.42 | NA | |
| Haller cell | 3.63 (1.02, 12.93) | 0.04* | 6.38 (0.53, 77.55) | 0.15 |
| Paradoxical MT | 0.31 (0.03, 3.17) | 0.30 | NA | |
| Everted UP | 1.00 (0.06, 16.69) | >0.99 | NA | |
| Sphenoid sinus and surrounding structures | ||||
| Onodi cell | 1.62 (0.53, 4.94) | 0.40 | NA | |
| Sphenoid lateral recess | 2.44 (0.89, 6.65) | 0.08 | NA | |
| Complete accessory septum of SS | 3.07 (1.05, 8.93) | 0.04* | 1.24 (0.27, 5.75) | 0.78 |
| Pneumatization type (sellar) | 10.00 (1.87, 53.48) | <0.01** | 8.96 (1.27, 63.19) | 0.03* |
References
1) Callejas CA, Douglas RG. Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy 2013;43(8):835–49.


2) Montone KT, Livolsi VA, Feldman MD, Palmer J, Chiu AG, Lanza DC, et al. Fungal rhinosinusitis: a retrospective microbiologic and pathologic review of 400 patients at a single university medical center. Int J Otolaryngol 2012;2012:684835.



3) Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope 2009;119(11):2275–9.


4) Shin JM, Baek BJ, Byun JY, Jun YJ, Lee JY. Analysis of sinonasal anatomical variations associated with maxillary sinus fungal balls. Auris Nasus Larynx 2016;43(5):524–8.


5) Costa F, Emanuelli E, Franz L, Tel A, Sembronio S, Robiony M. Fungus ball of the maxillary sinus: retrospective study of 48 patients and review of the literature. Am J Otolaryngol 2019;40(5):700–4.


6) Tomazic PV, Dostal E, Magyar M, Lang-Loidolt D, Wolf A, Koele W, et al. Potential correlations of dentogenic factors to the development of clinically verified fungus balls: a retrospective computed tomography-based analysis. Laryngoscope 2016;126(1):39–43.


7) Doo JG, Min HK, Choi GW, Kim SW, Min JY. Analysis of predisposing factors in unilateral maxillary sinus fungal ball: the predictive role of odontogenic and anatomical factors. Rhinology 2022;60(5):377–83.


8) Hwang SH, Kang JM, Cho JH, Kim BG. What is the relationship between the localization of maxillary fungal balls and intranasal anatomic variations? Clin Exp Otorhinolaryngol 2012;5(4):213–7.



9) Tsai TL, Guo YC, Ho CY, Lin CZ. The role of ostiomeatal complex obstruction in maxillary fungus ball. Otolaryngol Head Neck Surg 2006;134(3):494–8.


10) Li L, Han D, Li S, Jr NRL. The association of nasal septal deviation with solitary sphenoidal fungus ball: retrospective analysis of 43 patients. J Otol Rhinol 2020;9(7):384.
11) Orhan I, Ormeci T, Bilal N, Sagiroglu S, Doganer A. Morphometric analysis of sphenoid sinus in patients with nasal septum deviation. J Craniofac Surg 2019;30(5):1605–8.


12) Li L, London NR Jr, Prevedello DM, Carrau RL. Endoscopic prelacrimal approach to lateral recess of sphenoid sinus: feasibility study. Int Forum Allergy Rhinol 2020;10(1):103–9.


13) Aksoy F, Yenigun A, Goktas SS, Ozturan O. Association of accessory sphenoid septa with variations in neighbouring structures. J Laryngol Otol 2017;131(1):51–5.


14) Gibelli D, Cellina M, Gibelli S, Oliva G, Termine G, Dolci C, et al. Prevalence of accessory septations of sphenoid sinus in pediatric population: applications to endoscopic sinus surgery. Anat Rec (Hoboken) 2020;303(8):2171–6.


15) Alsaied AS. Paranasal sinus anatomy: what the surgeon needs to know. In Gendeh BS, editor. Paranasal Sinuses [Internet]. London: IntechOpen; 2017 [cited 2023 Dec 1]. Available from: https://doi.org/10.5772/intechopen.69089.

16) Eberhardt JA, Torabinejad M, Christiansen EL. A computed tomographic study of the distances between the maxillary sinus floor and the apices of the maxillary posterior teeth. Oral Surg Oral Med Oral Pathol 1992;73(3):345–6.


17) Basurrah M, Kim DH, Lee IH, Kim SW, Kim SW. Effects of dental factors on fungal sinusitis. ORL J Otorhinolaryngol Relat Spec 2022;84(4):309–14.


18) Grosjean P, Weber R. Fungus balls of the paranasal sinuses: a review. Eur Arch Otorhinolaryngol 2007;264(5):461–70.


19) Lim HS, Yoon YH, Xu J, Kim YM, Rha KS. Isolated sphenoid sinus fungus ball: a retrospective study conducted at a tertiary care referral center in Korea. Eur Arch Otorhinolaryngol 2017;274(6):2453–9.


20) Alkire BC, Bhattacharyya N. An assessment of sinonasal anatomic variants potentially associated with recurrent acute rhinosinusitis. Laryngoscope 2010;120(3):631–4.


21) Başer E, Sarioğlu O, İdil M, Çukurova İ, Arslan İB. Effect of sphenoid sinus volume and pneumatization type on isolated chronic sphenoid sinusitis fungi and polyps. Tr-ENT 2020;30(2):58–65.

22) Illing E, Schlosser RJ, Palmer JN, Curé J, Fox N, Woodworth BA. Spontaneous sphenoid lateral recess cerebrospinal fluid leaks arise from intracranial hypertension, not Sternberg’s canal. Int Forum Allergy Rhinol 2014;4(3):246–50.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print