Insufficiency of Laboratory Data in Reflecting Allergic Rhinitis Severity Based on the Allergic Rhinitis and Its Impact on Asthma Guideline in Korean Patients
Article information
Abstract
Background and Objectives
This retrospective study, conducted at a single tertiary medical center, aimed to investigate the correlation between the severity of allergic rhinitis (AR) based on subjective symptoms and the severity assessed through laboratory data.
Methods
In total, 584 patients who were diagnosed with AR by a multiple-allergen simultaneous test were included. Patients were classified into four groups according to the Allergic Rhinitis and its Impact on Asthma (ARIA) classification guideline. The visual analog scale (VAS) score for overall discomfort and laboratory parameters, including the serum total immunoglobulin E (IgE) level and peripheral blood eosinophil count, were evaluated in all patients. An analysis was conducted to examine the differences in VAS scores and laboratory findings among the four groups. Additionally, the correlations between the laboratory findings and VAS score were analyzed.
Results
The serum total IgE level and the percentage and count of peripheral blood eosinophils showed no significant differences among the groups. However, the VAS score for overall discomfort exhibited notable between-group differences. The average VAS score was 6.14 (95% confidence interval 5.94–6.34) in the overall group. The mean scores of each group showed a noticeable increasing trend from the mild intermittent group to the mild persistent, moderate to severe intermittent, and moderate to severe persistent groups (p<0.001), although there was no clear correlation between the increase in VAS scores and laboratory parameters.
Conclusion
Neither the symptom-based ARIA guideline nor the VAS score correlated with the AR laboratory test measurements. The current laboratory data alone may not be sufficient to reflect the severity of AR based on subjective symptoms.
INTRODUCTION
Allergic rhinitis (AR) is one of the most common chronic conditions and is increasingly prevalent in high-income countries, with some regions experiencing a prevalence of up to 50% [1]. It is caused by an immunoglobulin E (IgE)-mediated immune response triggered by exposure to inhaled allergens. The major symptoms of AR include sneezing, nasal itching, watery rhinorrhea, and nasal congestion [2]. Allergic rhinitis can also have a considerable impact on the quality of life, leading to decreased social activity, impaired school performance, and reduced productivity, especially in moderate to severe cases [3,4]. Thus, it is important to diagnose and manage this health problem properly.
For chronic rhinosinusitis (CRS), which is another common disease of rhinology, the new concept of endotype classification has recently been introduced. It has been reported that the disease can have different phenotypes and treatment outcomes depending on its endotype. When diagnosing and treating type II CRS, which is often eosinophilic CRS caused by type II inflammation, physicians use both physical indicators, such as nasal polyps, as well as laboratory measurements, including serum total IgE level, peripheral blood eosinophil counts, and tissue eosinophil counts, to determine the severity of the disease and make treatment decisions. Likewise, AR is mainly caused by type II inflammation. It would be worth exploring if there are any substantial differences in type II–related lab tests, such as serum total IgE level and blood eosinophil counts, with the progression of AR severity [5,6].
The Allergic Rhinitis and its Impact on Asthma (ARIA) guideline is commonly used to classify AR based on its duration and severity. According to a previous study, a longer duration of AR symptoms is significantly associated with a higher blood eosinophil count [7]. However, few studies have investigated serum total IgE levels and peripheral blood eosinophils in each ARIA group.
The newly revised ARIA guideline proposes a methodology that uses a visual analog scale (VAS) for scoring the overall discomfort level in AR and incorporating it into treatment to improve disease control from the patient’s perspective. The guideline introduces an algorithm that uses the VAS score to select the initial pharmacotherapy and determine step-up/-down treatment [8,9]. Based on this trend, analyzing the correlations of VAS scores with laboratory parameters would also be meaningful.
This study classified AR based on the ARIA guideline or VAS score for overall discomfort. We examined whether the ARIA guideline or VAS score reflect the differences in AR severity observed in laboratory data. Ultimately, we aimed to investigate the correlation between the severity of AR based on subjective symptoms and the severity assessed through laboratory data.
METHODS
Subjects
Patients with AR who visited the Department of Otorhinolaryngology at Severance Hospital from June 2020 to June 2023 were included. All patients were asked about their medical history and underwent a physical examination. Diagnosis of AR was performed with the multiple-allergen simultaneous test (MAST) for 62 inhalant allergens (AdvanSure Allostation Smart II; LG Life Science, Seoul, Korea). On the MAST, subjects were diagnosed with AR if the serum-specific IgE was positive for one or more inhalant allergens. Patients who had endoscopic functional sinus surgery due to combined CRS were excluded to eliminate the effect of other combined nasal inflammatory diseases on symptoms, except for AR. All subjects responded to a questionnaire for allergic symptoms, including the VAS for overall discomfort, and were classified into sub-groups according to the ARIA guideline. This study was approved by the Institutional Review Board of Severance Hospital, Seoul, Korea (IRB No. 4-2023-0992) and the need for informed consent was waived.
ARIA classification
Based on the ARIA guideline, we classified patients whose duration of AR was less than 4 days per week or 4 weeks per year as the intermittent group and those with a duration of more than 4 days per week and 4 weeks per year as the persistent group. Then, they were classified as mild or moderate to severe based on the severity of symptoms. The patients who had at least one of four items—sleep disturbance; impairment of daily activities, including leisure and/or sport; impairment of school or work; or troublesome symptoms—were classified as the moderate to severe group. The remaining patients were categorized as the mild group [10,11].
Questionnaire for the AR symptoms
The questionnaire included 13 items assessing the severity of symptoms on a 5-point scale, two items regarding duration, and a VAS for the overall impact of the symptoms on the patients (Supplementary Material in the online-only Data Supplement).
Laboratory tests
We measured the serum total IgE level and the percentage and count of peripheral blood eosinophils for each patient. We then compared the laboratory findings between each ARIA group and analyzed the correlations between the laboratory results and VAS scores.
Statistical analysis
The statistical analysis was performed with SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA) using appropriate methods as indicated in each figure legend and the results. The following statistical tests were used: the Kruskal–Wallis test, Mann–Whitney test, chi-square test, Fisher exact test, Jonckheere–Terpstra test, Spearman rank correlation, and Kendall tau test.
RESULTS
In total, 584 patients were included in the test. The mild intermittent group accounted for 15.1% of patients (88 patients), the mild persistent group 3.3% (19), the moderate to severe intermittent group 27.6% (161), and the moderate to severe persistent group 54.1% (316) (Table 1).
The serum total IgE level was not significantly different among the groups. The average value of the results was 320.3 kU/L. It was 296.9 kU/L in the mild intermittent group, 452.5 kU/L in the mild persistent group, 288.4 kU/L in the moderate to severe intermittent group, and 335.1 kU/L in the moderate to severe persistent group (Fig. 1A).
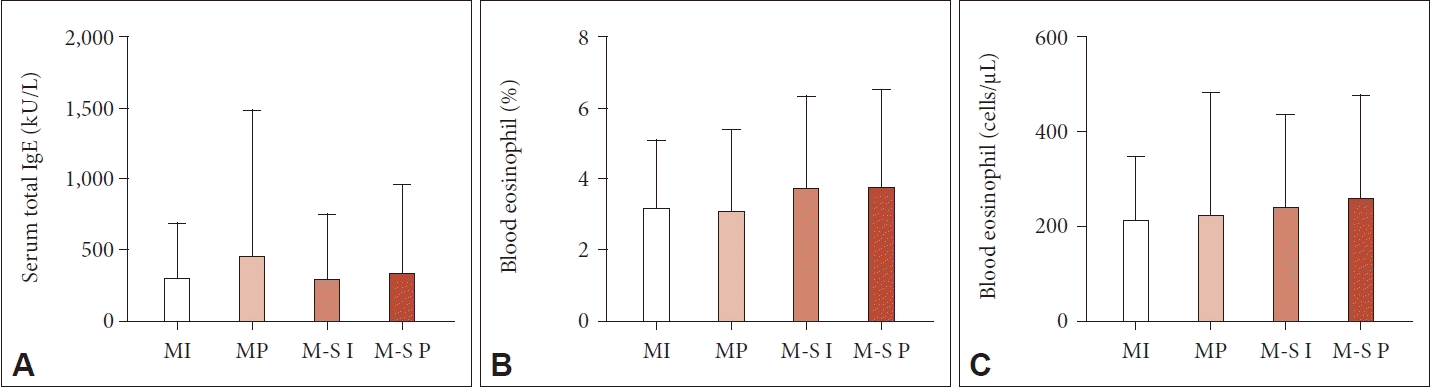
Serum total IgE and peripheral blood eosinophil count for each ARIA group. A: For the serum total IgE level, there was no significant difference between groups. B and C: The blood eosinophil count also showed no significant difference between groups. The Kruskal– Wallis test was applied for comparisons between groups (p>0.05). ARIA, Allergic Rhinitis and its Impact on Asthma; MI, mild intermittent; MP, mild persistent; M-S I, moderate to severe intermittent; M-S P, moderate to severe persistent.
Furthermore, the percentage and count of peripheral blood eosinophils were also not significantly different among the groups. The average blood eosinophil percentage (count) was 3.64% (245.0 cells/μL) overall, 3.16% (211.6 cells/μL) in the mild intermittent group, 3.09% (223.7 cells/μL) in the mild persistent group, 3.72% (240.0 cells/μL) in the moderate to severe intermittent group, and 3.77% (258.1 cells/μL) in the moderate to severe persistent group (Fig. 1B and C).
The correlation between the total serum IgE level and blood eosinophil count was analyzed with the Spearman rank test and the Kendall tau test. These two laboratory results showed a positive correlation (p<0.001), but the relationship was minor (ρ=0.191, τ=0.131).
The VAS score for overall discomfort was 6.14 (95% confidence interval, 5.94–6.34) on average. The average group scores were as follows: 2.08 (1.84–2.34) for the mild intermittent group, 4.74 (3.84–5.63) for the mild persistent group, 5.84 (5.56–6.12) for the moderate to severe intermittent group, and 7.51 (7.34–7.69) for the moderate to severe persistent group. The scores between each group showed significant differences (Kruskal–Wallis test p<0.001; post-hoc Mann–Whitney test p<0.05) (Fig. 2), and a significantly noticeable increasing trend was observed from the mild intermittent to mild persistent, moderate to severe intermittent, and moderate to severe persistent groups (Jonckheere–Terpstra test p<0.001). In each symptom, a similar trend for significant differences was observed (Jonckheere–Terpstra test p<0.001). Detailed information on symptom scores in the groups is presented in Supplementary Table 1 (in the online-only Data Supplement).

Visual analog scale (VAS) scores for overall discomfort caused by allergic rhinitis. All groups have significant differences each other (Kruskall–Wallis, p<0.001; Mann–Whitney, p<0.05). The VAS score showed a tendency to increase from the MI to the M-S P group (M-S P>M-S I>MP>MI; Jonckheere–Terpstra, p<0.001). MI, mild intermittent; MP, mild persistent; M-S I, moderate to severe intermittent; M-S P, moderate to severe persistent.
We also analyzed the correlations between the VAS score and laboratory findings using Spearman rank correlations and the Kendall tau. There was no significant relationship between the VAS score and serum total IgE level (p>0.05). The VAS score and blood eosinophil count showed a significantly positive correlation (p<0.05). However, the magnitude of their relationship was minimal (ρ=0.093, τ=0.067).
DISCUSSION
Among the groups classified based on the ARIA guideline, the moderate to severe persistent group was the most common (54.1%), followed by the moderate to severe intermittent group (27.6%), the mild intermittent group (15.1%), and the mild persistent group (3.3%). This order of proportions is consistent with a previous European study [12]. Additionally, as is well known, house dust mites were the most common allergen for all the groups (Table 1). Regarding the laboratory findings for the total serum IgE and blood eosinophil count, there were no significant differences observed among the ARIA groups. There was a statistically significant positive correlation between the two results, but the correlation was found to be weak. In contrast, the VAS scores showed significant differences between each group, with an increasing trend from the mild intermittent group to the moderate to severe persistent group, which is in line with the results of a study published in 2007 [12]. This study, which aimed to evaluate whether the VAS score could assess the severity of AR in 2,908 patients, confirmed its statistical significance. According to the results of the study, the VAS scores for mild and moderate/severe patients could be divided using a cut-off level of 5 cm: mild intermittent (3.5; interquartile range, 2.4–5.0 cm), mild persistent (4.5; interquartile range, 3.2–5.6 cm), moderate to severe intermittent (6.7; interquartile range, 5.3–7.7 cm), and moderate to severe persistent (7.2; interquartile range, 6.1–8.2 cm) [13]. Despite exploring the relationship between VAS scores reflecting AR severity and lab findings, no prominent correlation was evident.
There are several possible reasons for the differences between the laboratory results and the severity of allergic symptoms. Allergic traits (atopic status) usually have systemic effects, impacting conditions such as atopic dermatitis and asthma, but AR exerts a more localized influence than other allergic conditions. Recent studies have focused on the concept of “local AR,” referring to AR without systemic sensitization (systemic sensitization presents as serum-specific IgE positivity or positivity on a skin prick test) [14,15]. Due to these local effects, the severity of AR might not be adequately reflected in systemic values, such as the total serum IgE and blood eosinophil count. To precisely understand the correlation between laboratory findings and the severity of AR, we may need to evaluate locally elevated eosinophil counts or locally produced IgE. Furthermore, the blood eosinophil count clinically shows a large variation depending on the test point, so it might not be suitable for routinely evaluating the disease status, especially as a single indicator.
As mentioned above, according to a study published in 2008 [7], the blood eosinophil counts of the persistent groups were significantly higher than those of the intermittent groups. The results of that study are somewhat inconsistent with the results of our study. We believe that those results are still up for debate. Our study indicates that relying on laboratory data alone may not be sufficient for reflecting the severity of AR based on the ARIA guideline, particularly in Korean patients. Consequently, it may be challenging to predict severity based on symptoms using current laboratory data.
This study has several limitations. It had a small sample size, and the analysis focused solely on the relationship between initial symptoms and initial laboratory data. To assess the effectiveness of laboratory data in predicting symptom severity, an additional evaluation would be needed to understand how the improvement in symptoms of AR during the follow-up period is related to changes in laboratory data. It is also worth noting that we diagnosed AR with MAST, which is considered a screening test, rather than immunoCAP or a skin prick test. While we opted for MAST due to its common use, simplicity, and cost-effectiveness, further investigations employing immunoCAP or skin prick tests would be necessary for a more precise analysis. Nevertheless, the study’s strength lies in evaluating the severity of AR using a simple VAS method and conducting additional analysis of its correlations with AR laboratory data.
In conclusion, the groups classified according to the ARIA guideline did not show any significant differences in serum total IgE and peripheral blood eosinophil counts. Furthermore, there was no clear correlation between higher VAS scores, which reflect the overall discomfort caused by AR, and serum total IgE or peripheral blood eosinophil levels. The results showed that serum total IgE and peripheral blood eosinophil levels were not significant indicators of AR severity based on symptoms.
When treating AR, it is crucial to use the symptom-based ARIA guideline or a VAS to determine treatment effectiveness and select appropriate medications. The patient’s subjective symptoms are the primary factor when assessing treatment efficacy.
However, the symptom-based scales did not correlate with the AR laboratory test measurements. Even when laboratory results are mild or severe, we may focus primarily on symptoms. In addition, the currently used systemic laboratory parameters of AR may not be suitable for assessing treatment effectiveness or forecasting the disease’s severity. There are limited studies assessing the correlation between laboratory tests for local AR, such as nasal-specific IgE and basophil activation tests, and symptom severity. Therefore, further investigations would be needed to conduct additional correlation analyses between these laboratory tests and the severity of AR based on symptoms.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.18787/jr.2023.00066.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Hae Eun Noh, Hyung-Ju Cho. Data curation: Hae Eun Noh. Formal analysis: Hae Eun Noh, Min-Seok Rha. Funding acquisition: Hyung-Ju Cho. Investigation: Hae Eun Noh, Yeonsu Jeong, Chang-Hoon Kim. Methodology: Hae Eun Noh, Yeonsu Jeong. Writing—original draft: Hae Eun Noh, Hyung-Ju Cho. Writing—review & editing: Hae Eun Noh, Hyung-Ju Cho.
Funding Statement
This study was supported by the “Team Science Award“ of Yonsei University College of Medicine (6-2021-0005).
Acknowledgements
None

