A Case of Bilateral Trigeminal Amyloidoma Diagnosed Through an Endoscopic Transsphenoidal Approach
Article information
Abstract
Amyloidosis is a systemic disease characterized by the accumulation of amyloid protein in multiple organs. Amyloidoma, in contrast, is an uncommon localized form of amyloidosis that presents as a single mass or tumor-like lesion. Primary amyloidoma in the central nervous system is rare, and only a few cases have been reported. Notably, the Gasserian ganglion is the most frequently affected site of amyloidoma in the central nervous system, and progressive trigeminal neuropathy is a characteristic finding. Among these cases, the bilateral occurrence of amyloidoma is exceedingly rare. In this report, we present the case of a 51-year-old woman diagnosed with bilateral trigeminal amyloidoma, confirmed by an endoscopic biopsy via the transsphenoidal approach.
INTRODUCTION
Amyloidosis is a cluster of diseases characterized by the extracellular accumulation of amyloid in various organs and tissues. Amyloid is composed of protein subunits that form distinctive structures of insoluble, anti-parallel, and β-pleated sheets [1]. The clinical features of amyloidosis vary depending on the type of amyloid and the location of its deposition. This condition can impact multiple organs and tissues systemically, or it may be localized to a single organ [2].
An amyloidoma is defined as a localized deposit of amyloid without a systemic manifestation. Amyloidomas have been described in the lung, liver, gastrointestinal tract, heart, skin, kidney, urinary tract, and endocrine tissues [3]. However, amyloidomas of the central nervous system are rare, especially with a bilateral presentation. Here, we present the case of a 51-year-old woman with bilateral trigeminal amyloidoma diagnosed through an endoscopic transsphenoidal approach, and we also review the relevant literature.
CASE REPORT
A 51-year-old woman with a history of atrial fibrillation and diabetes mellitus presented at a clinic, reporting bilateral facial numbness that had persisted for a year. A neurologist assessed her hypoesthesia, which was present in both trigeminal nerve distributions. Her symptoms began abruptly in the perioral area and eventually spread to her entire face. She also described an electric-like tingling sensation in her face, triggered by touch, and a slight malocclusion of the jaw, which resulted in drooling and mild dysarthria. Neurological examinations revealed a loss of sensation in the distribution of the bilateral trigeminal nerves, with the severity being particularly pronounced on the left side. The patient also exhibited reduced mastication power on the left side and weakness in closing her left eye. Other cranial nerve examinations yielded no remarkable findings.
Magnetic resonance imaging (MRI) revealed a heterogeneously enhancing soft tissue mass-like lesion along the bilateral course of the trigeminal nerve at the Meckel’s cave, foramen rotundum, and foramen ovale. The lesion on the left side was slightly larger than that on the right, extending more extensively towards the foramen ovale. This resulted in fatty atrophy of the left masticatory muscle group, possibly due to denervation (Fig. 1). The preoperative differential diagnosis of this lesion included immunoglobulin G4-related disease, inflammatory pseudotumor, granulomatous diseases, such as sarcoidosis, lymphoma, or perineural spread from a head and neck malignancy. A preoperative paranasal sinus computed tomography (CT) examination confirmed these radiologic findings (Fig. 2). The patient was then referred to the otorhinolaryngology-head and neck surgery department for a surgical biopsy.
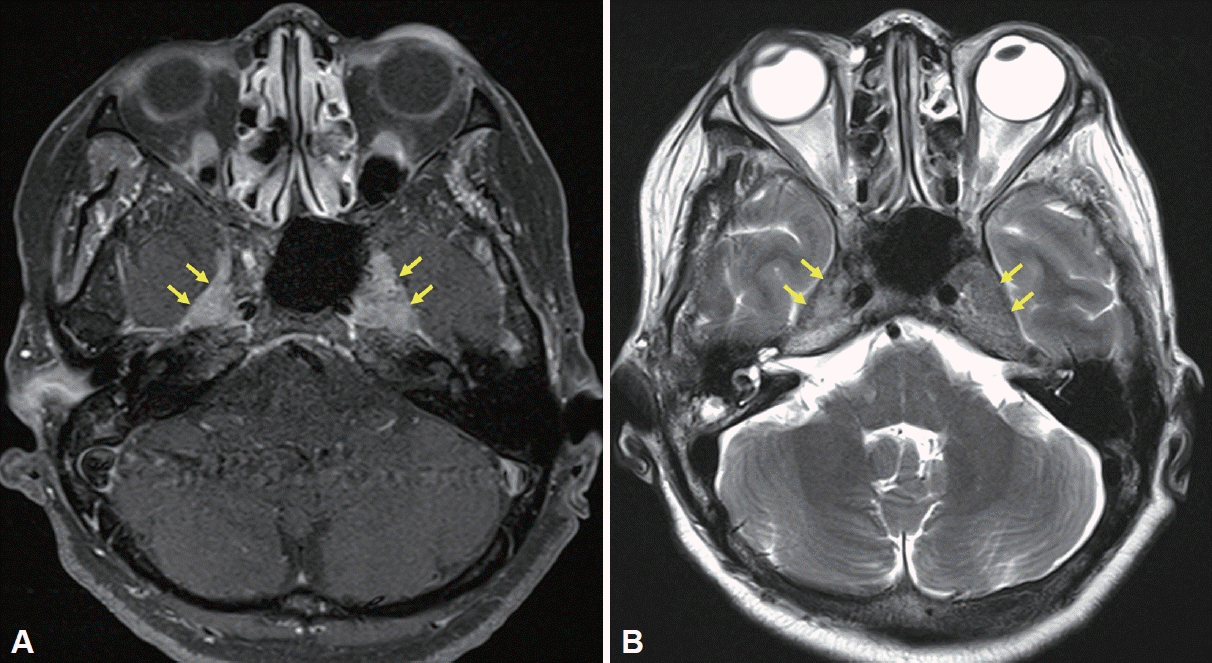
Magnetic resonance imaging of the bilateral Meckel’s cave. A: Axial contrast-enhanced T1-weighted image displaying involvement of bilateral Meckel’s cave (yellow arrows). B: T2-weighted image showing iso- to low-signal intensity (yellow arrows).
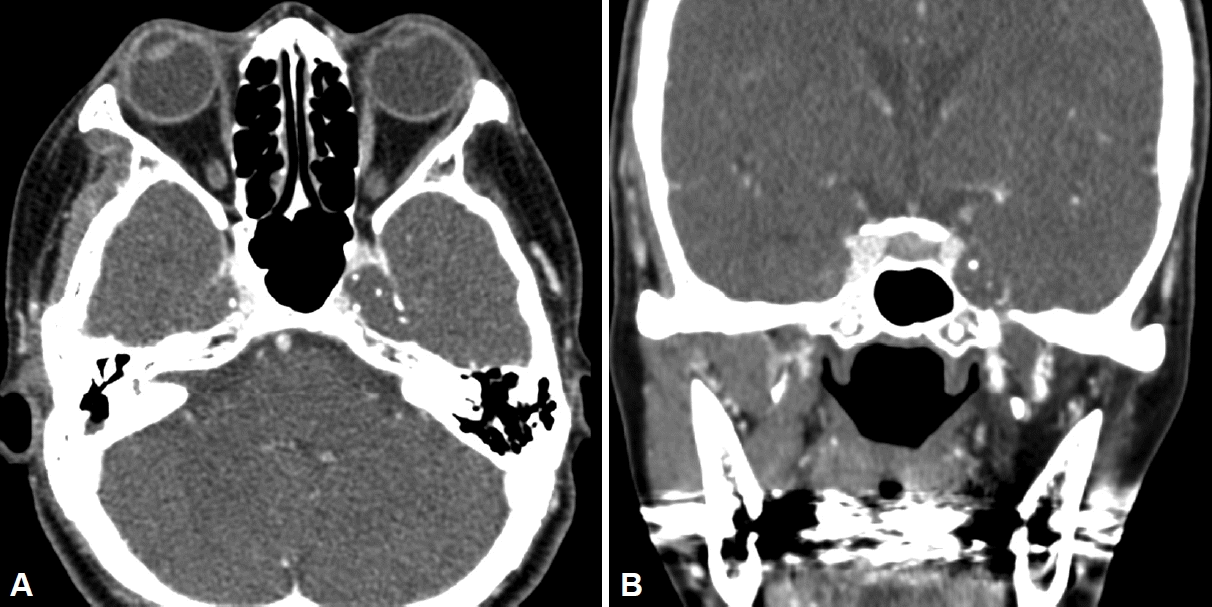
Paranasal sinus contrast-enhanced computed tomography scan showing a poorly enhancing soft-tissue lesion in the bilateral Meckel’s cave. A: Axial view. B: Coronal view.
The patient underwent a diagnostic biopsy using an endoscopic transsphenoidal approach under general anesthesia. For the binostril approach, a modified Killian incision was made on the right side, while a rescue flap was created on the left side to ensure a broader field of view. Following a posterior septectomy, the rostrum was drilled out and the anterior wall of the sphenoid sinus was excised. The mass was located using navigation, after which the mucosa covering the left sphenoid sinus lateral wall was removed. The bony wall was then excised using a drill and curette to expose the tumor located in the inferomedial area of the cavernous sinus. However, the frozen biopsy revealed only collagen tissue, with no inflammatory or tumor cells present. As a result, an extracapsular dissection was performed to biopsy a deeper site. Multiple biopsy samples were collected and sent for pathological examination. During the procedure, cerebrospinal fluid leakage occurred. To reconstruct the defect site, a nasoseptal flap was designed and harvested from the incision site of the left rescue flap. The flap was positioned along the lateral side of the sphenoid sinus to prevent kinking or folding of the pedicle.
The patient was restricted to bed rest for 24 hours after surgery. Postoperative sellar MRI and CT on the day after surgery revealed no significant abnormalities. The patient was discharged 3 days after surgery without significant complications. The final pathological examination showed a substantial deposition of hypocellular eosinophilic material when viewed under light microscopy, which is consistent with a diagnosis of amyloidosis (Fig. 3).

Hematoxylin and eosin-stained histologic sections demonstrate abundant amorphous eosinophilic material suspicious for amyloid.
Postoperatively, the patient was referred to the oncology department to rule out systemic amyloid involvement. Additional studies such as serum and urine protein electrophoresis and urinalysis showed no evidence of systemic amyloidosis, and the patient was diagnosed with isolated intracranial amyloidoma. We planned to monitor any changes in the patient’s symptoms through regular follow-up appointments. Five months postbiopsy, the patient reported difficulties with mastication, in addition to the previously noted hypoesthesia. We sought advice from a dentist, and the patient is currently on a regimen of medications, including neuropathic pain modulators. If the symptoms persist and prove resistant to medical treatment, we will consider conducting a follow-up MRI and potentially initiating radiotherapy.
DISCUSSION
Amyloidosis refers to the localized or systemic deposition of abnormal protein material composed of fibrils [4]. It is categorized as primary when no underlying disease is present, and secondary when associated with an underlying disease such as plasmacytoma, rheumatoid arthritis, or chronic renal failure [5]. The term amyloidoma is used when amyloidosis is confined to a specific organ, without any evidence of systemic involvement. Isolated bilateral trigeminal amyloidomas are uncommon, and their etiology remains poorly understood [6]. Numerous studies have suggested that the kappa and lambda light chains, derived from immunoglobulins and likely produced by clonal plasma cells, are the primary source of most cerebral amyloidomas [7,8].
Previous reports indicate a higher prevalence of the condition in females, with the age at diagnosis typically ranging from 27 to 70 years, and a mean age of 47 years [2]. The most frequently reported symptoms are pain and numbness in at least two branches of the trigeminal nerve, and in some instances, weakness in the masticatory muscles has also been noted [9,10]. Imaging results are variable and non-specific, often revealing a mass-forming lesion within Meckel’s cave or a slight enlargement of the trigeminal nerve. Lesions usually exhibit intense homogeneous contrast enhancement on MRI scans, while CT results are either normal or isodense with homogenous contrast enhancement [11].
Amyloidomas are frequently indeterminate on radiographic images, necessitating a histopathological examination for a definitive diagnosis [2]. Under light microscopic examination, amyloid presents as an eosinophilic amorphous hyaline extracellular substance, exhibiting characteristic apple-green birefringence when viewed under polarized light and stained with Congo red. Neurosurgeons most commonly perform craniotomies for surgical biopsies [9]. In the present case, however, we performed a biopsy through an endoscopic approach, which provides superior cosmetic results and lessens morbidity. Despite encountering intraoperative cerebrospinal fluid leakage, we successfully reconstructed the defect site using a nasoseptal flap, without any significant complications. Following pathologic confirmation, we conducted various tests to rule out systemic amyloidosis. For localized amyloidoma, several treatment options can be considered, including surgical resection, medical therapy, and radiation therapy, although there is no consensus on the best treatment approach [2].
Trigeminal amyloidoma is typically a slow-growing condition; it is often diagnosed several years after the onset of symptoms, and it is generally classified as benign [9]. However, due to its low incidence and the limited number of published reports, the clinical progression of trigeminal amyloidoma remains largely unknown. Some cases have demonstrated improved sensation post-surgery, as documented in Gültaşli et al. [11]. Conversely, there have been instances where pain was alleviated after surgery, but facial anesthesia persisted [12,13]. Additionally, there was a case in which the patient’s symptoms remained stable after radiation therapy following a biopsy [2]. Therefore, further research, incorporating long-term follow-up results, is necessary to establish appropriate management strategies.
Notes
Ethics Statement
Thee Institutional Review Board of the Samsung Medical Center approved this study (IRB No. SMC 2023-05-142-001) and the informed consent was obtained.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Gwanghui Ryu who is on the editorial board of the Journal of Rhinology was not involved in the editorial evaluation or decision to publish this article. The remaining author has declared no conflicts of interest.
Author Contributions
Conceptualization: Gwanghui Ryu. Data curation: Song I Park. Methodology: Gwanghui Ryu. Supervision: Gwanghui Ryu. Writing—original draft: Song I Park. Writing—review & editing: Gwanghui Ryu.
Funding Statement
None
