|
 |
| J Rhinol > Volume 30(3); 2023 |
|
Abstract
Background and Objectives
Methods
Results
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Author Contributions
Conceptualization: Jeong-Hee Choi, Seok Jin Hong. Data curation: Sung Hun Kang, Jeong-Hee Choi. Formal analysis: Sung Hun Kang, Sun-Ju Byeon, Seok Jin Hong. Investigation: Joon-Pyo Hong, Hyunjoo Lee. Methodology: Joon-Pyo Hong, Sung Hun Kang, Sun-Ju Byeon. Supervision: Jinah Chu, Seok Jin Hong, Kyung Chul Lee. Validation: Jinah Chu, Sun-Ju Byeon, Seok Jin Hong. Visualization: Sung Hun Kang, Seok Jin Hong. Writing—original draft: Joon-Pyo Hong. Writing—review & editing: Jinah Chu, Seok Jin Hong.
Fig. 1.
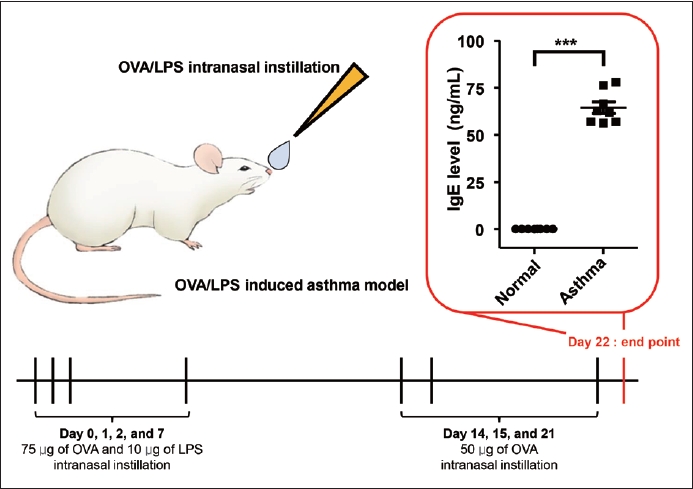
Fig. 2.

Fig. 3.
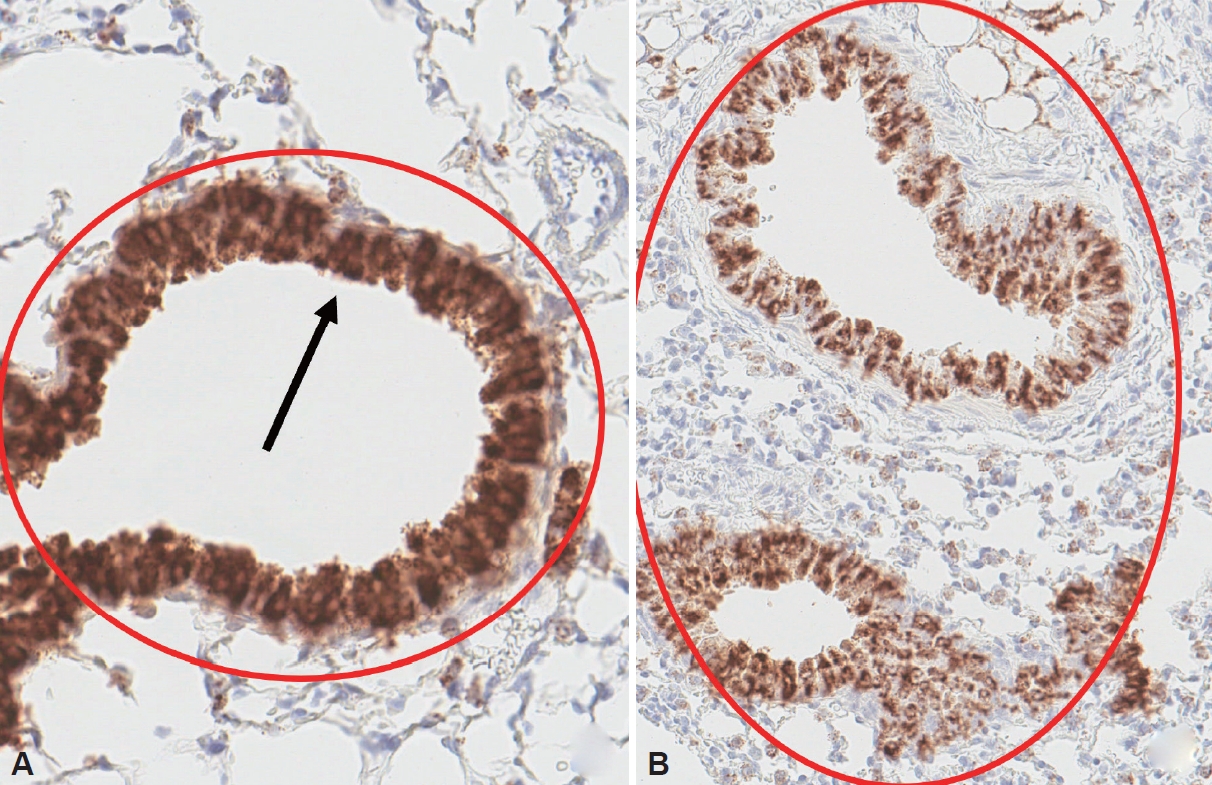
Fig. 4.

Table 1.
| Variables | Normal | Asthma | p |
|---|---|---|---|
| Serum zonulin (ng/mL) | 47.81±6.55 | 65.88±21.86 | 0.042* |
| Cytokine (pg/mL) | |||
| IL-1β | 1.67±1.53 | 10.39±8.28 | 0.214 |
| IL-4 | 0.82±0.15 | 1.72±0.44 | 0.019* |
| IL-6 | 5.20±4.31 | 36.61±17.17 | 0.038* |
| IL-10 | 4.65±0.84 | 5.91±3.10 | 0.486 |
| IL-13 | 71.29±7.20 | 93.99±19.38 | 0.341 |
| IL-25/IL-17E | 964.12±156.38 | 1,200.14±503.71 | 0.424 |
| IL-33 | 13.31±11.71 | 21.20±6.70 | 0.253 |
| TNF-α | 3.26±1.21 | 4.61±4.10 | 0.672 |
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,136 View
- 17 Download
- Related articles
-
The Relationship between Obstructive Sleep Apnea and Metabolic Syndrome in Adult2016 November;23(2)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print