A Survey on Biologics for the Treatment of Chronic Rhinosinusitis With Nasal Polyps Among Members of the Korean Rhinologic Society
Article information
Abstract
Background and Objectives
In 2021, biologics were approved for treating chronic rhinosinusitis with nasal polyps (CRSwNP) in Korea. However, CRS is a heterogeneous disease, and its characteristics are thought to differ between Western and Korean populations. This study aimed to evaluate the experiences of members of the Korean Rhinologic Society during the first year of biologic usage for the treatment of nasal polyps.
Methods
An anonymous survey consisting of 15 items was conducted from November to December 2021. The survey included questions about participant demographics, use of biologics for treating CRSwNP, and expectations regarding the effectiveness of biologics for treating CRSwNP.
Results
In total, 44 members participated in the survey. Approximately half of the respondents were in their 40s (50.0%) and had 5–9 years of clinical experience as otorhinolaryngologists (47.7%). The majority of participants held academic positions (95.4%). About half of them worked in Gyeonggi Province. The utilization of biologics did not differ significantly based on clinical experience (p=0.192). When asked about the factors considered for prescribing biologics, the most common reason was recurrence of polyps after surgery (87.2%). The most frequent reason for discontinuing biologics was cost (48.6%). When asked about the extent to which they expected that the availability of biologics for CRSwNP treatment would reduce endoscopic sinus surgery (ESS), 45.5% of members expected a reduction of approximately 10%–29%. In addition, 20.5% expected a reduction of 50% or more. However, 61.4% expected a reduction of less than 10% in primary ESS. In addition, most respondents (93.2%) agreed with the need for Korea-specific guidelines for biologic treatment.
Conclusion
There are discrepancies between the current guidelines for biologic treatment of CRSwNP and the reality of the situation, highlighting the need for the development of Korea-specific guidelines.
INTRODUCTION
The prevalence of chronic sinusitis is 7%–8% in Korea [1]. Chronic sinusitis is a heterogeneous disease defined as the presence of two or more symptoms persisting for a minimum of 12 weeks, with at least one being nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip) and optional symptoms of facial pain/pressure and reduction or loss of smell [2]. In the past, chronic rhinosinusitis (CRS) was classified only based on the phenotype, as CRS with nasal polyps (CRSwNP) or CRS without nasal polyps (CRSsNP) [3]. However, in EPOS 2020 (European Position Paper on Rhinosinusitis and Nasal Polyps 2020), the classification was revised to categorize CRS based on cause into primary and secondary, anatomical extent into unilateral and bilateral, and endotype into type 2 and non-type 2 [2]. The endotype is emphasized because it is associated with different medical treatment strategies and outcomes [2]. Until recently, repeated revision surgery, reboot surgery, and steroid therapy were attempted for patients with severe type 2 CRS who experienced persistent recurrence even after surgery [4]. However, in 2021, biologics were introduced, providing a new weapon in the treatment of CRSwNP [5]. Therefore, the importance of the endotype has been increasingly emphasized, and numerous recent studies have aimed to understand treatment using clustering techniques based on tissue cytokines [6-8]. According to these studies, the distribution of nasal polyps based on endotypes in East Asia, including Korea and China, is different from that in Japan and the West [6-10]. This raises concerns about treating patients based on research results from Japan or the West. In the present study, we examined the initial experiences and expectations of biologics among members of the Korean Rhinologic Society.
METHODS
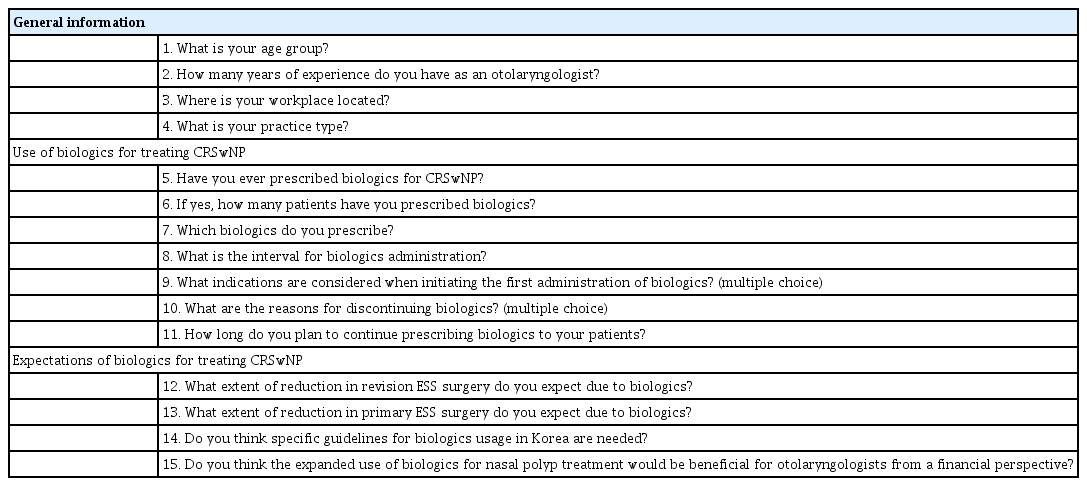
A survey was conducted from November to December 2021 among otolaryngologists who are affiliated with the Korean Rhinologic Society, and an online survey was sent out by e-mail and messenger. The survey questionnaire included demographic information of responders, such as age, number of years in practice, practice region, and practice type. It also gathered information regarding participants’ experience with the usage of biologics, including their experience using biologics, the number of patients prescribed biologics, specific biologics in use, intervals of usage, considered indications, reasons for discontinuation, and intended duration of usage. Additionally, participants were asked about their expectations regarding biologics, including the expected reduction rates of revision and primary endoscopic sinus surgery (ESS), as well as their perceived need for Korean guidelines (Table 1). This study received approval from the Institutional Review Board of Samsung Medical Center (IRB No. 2023-08-078), and the requirement for informed consent was waived.
Statistical analysis
Categorical variables were presented as number (percentage). Differences in experience using biologics according to the number of years in practice were compared using the chisquare test. The number of patients using biologics in comparison to the number of years the prescribing physician had been in practice was assessed using Spearman correlation coefficients. All statistical analyses were performed using R (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
RESULTS
Participant demographics
In total, 44 rhinology specialists responded to the survey. Half of them (50.0%) were in their 40s, 25% were in their 50s, 18.2% were in their 30s, and 6.8% were in their 60s. The most frequent duration of clinical practice as a rhinology specialist was 5 to 9 years (47.7%). Furthermore, 29.5% reported a practice duration of less than 5 years, while 20.5% had a practice duration of 10 to 19 years. A small percentage (2.3%) reported a practice duration of 20 years or more. Among the responding physicians, the highest proportion of participation was observed from Gyeonggi Province, accounting for 52.3%. The next highest proportion of responding physicians was in the Chungcheong region, representing 22.7%. The Gyeongsang and Jeolla regions accounted for 13.6% and 6.8% of responses, respectively, while the Gangwon region represented 4.5%. Most respondents were in academic positions (95.4%) (Table 2).
Use of biologics for treating CRSwNP
Among all respondents, approximately 86.4% had used biologics for treating CRSwNP. The usage of biologics did not differ by clinical experience (p=0.192). Most of the clinicians prescribed biologics to 10 patients or fewer (93.8%), and the number of patients prescribed biologics was not correlated with clinical experience (rho=-0.021, p=0.896). Regarding the types of biologics used, 71.1% of clinicians had experience prescribing dupilumab, while 10.5% had experience prescribing omalizumab, and 18.4% had experience with both.
In the survey questionnaire listing the factors considered as indications or reasons for medication use, the most common was the recurrence of polyps after surgery, followed in descending order by a reduced sense of smell; asthma; or evidence of a type 2 response, such as blood eosinophils and tissue eosinophils (Fig. 1).
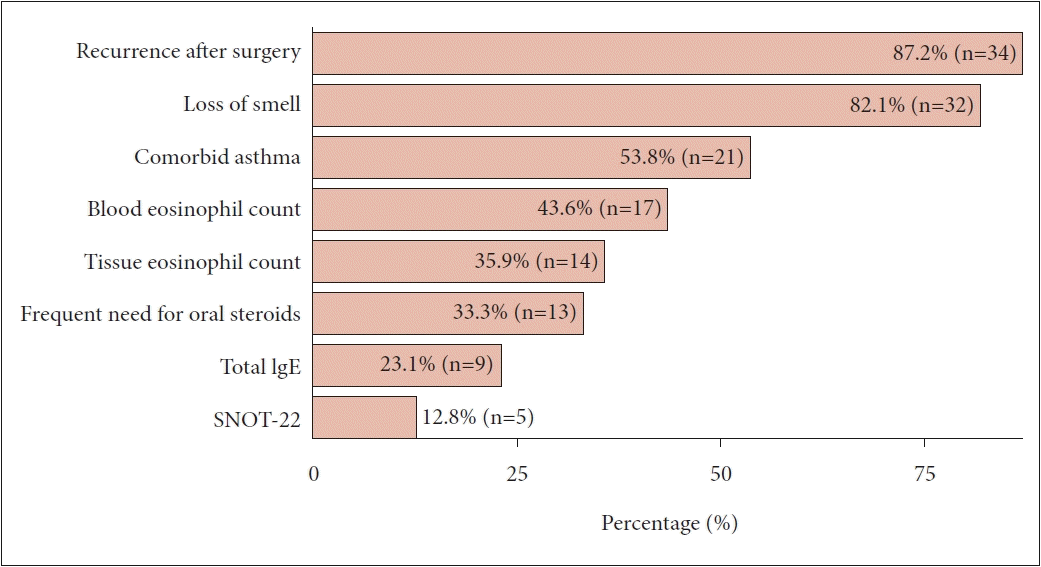
Respondents’ selection of indications for initiating biologics. IgE, immunoglobulin E; SNOT-22, Sino-Nasal Outcome Test.
The most common reported reason for discontinuing biologics was cost (48.6%), followed by lack of symptom improvement (21.6%). In addition, there were two cases where treatment was discontinued due to complete symptom resolution. Among other reasons for discontinuation, newly diagnosed tuberculosis, pregnancy, follow-up loss, side effects, and joint pain each accounted for one case, making up 5.4% each (Fig. 2). Furthermore, 27.0% of respondents indicated that they had not yet discontinued biological treatment (Fig. 2), 31.6% considered maintaining biologics for approximately 6–12 months, and 28.9% considered using it for more than 1 year. Additionally, 23.7% indicated that they planned to continue the therapy until the patient requested to stop (Table 3).
Expectations of biologics for treating CRSwNP
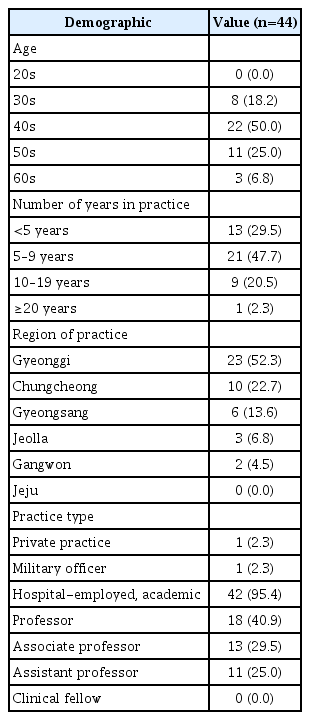
In response to an item regarding to what extent participants expected that the use of biologics for CRSwNP treatment would lead to a reduction in revision ESS, 45.5% stated that they expected a decrease of 10%–29%. Additionally, 20.5% had expectations for a decrease of 50% or more. However, 61.4% expected a decrease of less than 10% in primary ESS. Furthermore, most (93.2%) participants agreed that there is a need for Korean guidelines regarding biological treatment (Table 4).
DISCUSSION
This study investigated the perceptions of biologics among members of the Korean Rhinology Society in the year after their introduction. Most physicians in academic positions showed interest and participated in the questionnaire, and early adoption of biologics was not related to the length of the physician’s clinical experience. More physicians prescribed dupilumab than omalizumab, which could be attributed to the delayed approval of omalizumab for nasal polyp treatment in Korea. However, omalizumab targets free total immunoglobulin E, while dupilumab targets interleukin-4 Rα, indicating a difference in their mechanisms of action [11,12]. Recent studies have focused on understanding the heterogeneous subgroups of CRS by clustering tissue cytokines, aiming to identify variations between Eastern and Western populations [6,7]. Moving forward, a classification of CRS based on tissue cytokines and use of real-world data regarding biologics could assist in the development of more specific indications for each biologic agent.
According to the EPOS/EUFOREA 2023 criteria, biological treatment should be considered first in patients with bilateral polyps who have previously undergone ESS [13]. Consistent with this, doctors from the Korean Rhinology Society also considered the most favorable indication for biologic therapy to be recurrence in patients who had previously undergone surgery. If the above conditions are met, five criteria are considered, and a biologic is prescribed if at least three of them are met: evidence of type 2 inflammation, systemic steroid dependence or contraindication to systemic steroids, significantly impaired quality of life, loss of smell, and presence of comorbid asthma. The results of the questionnaire showed that, among the five criteria to be considered, loss of smell, the diagnosis of comorbid asthma, and evidence of type 2 inflammation were most often listed as indications. In addition, 43.6% of the respondents also considered the blood eosinophil level before prescribing biologics, and 35.9% of the respondents considered the tissue eosinophil level (Fig. 1). The EPOS/EUFOREA response criteria recommend discontinuing biologics if there is no improvement in any of the five symptoms after 6 months or 1 year [13]. However, in our experience, the most common reason for discontinuing medication is the high cost. Furthermore, more than one-third of physicians considered continuing biologics for one year. Therefore, it is important to prescribe biologics under proper indications and to continue prescribing them with careful consideration of cost-effectiveness. This approach will ensure that patients receive the maximum benefit from biologics [5].
This study is limited by the incomplete participation of members of the Korean Rhinologic Society, and most respondents were physicians in academic positions. Therefore, further investigation involving a larger number of otolaryngologists is needed. Nevertheless, our research confirmed discrepancies between the current guidelines and the real-world situation and will serve as a framework for developing Korean guidelines for the use of biologics to treat CRSwNP.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Gwanghui Ryu who is on the editorial board of the Journal of Rhinology was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: all authors. Data curation: Gwanghui Ryu, Shin Hyuk Yoo. Formal analysis: Hyunkyung Cha, Gwanghui Ryu. Investigation: all authors. Methodology: Gwanghui Ryu, Shin Hyuk Yoo, Ji-Hun Mo. Visualization: Hyunkyung Cha. Writing—original draft: Hyunkyung Cha, Gwanghui Ryu. Writing—review & editing: Shin Hyuk Yoo, Ji-Hun Mo.
Funding Statement
This paper was supported by fund of Korean Rhinologic Society in 2021.