Effects of a Proton-Pump Inhibitor on Postnasal Drip Symptoms in Patients With Laryngopharyngeal Reflux
Article information
Abstract
Background and Objectives
Laryngopharyngeal reflux (LPR) is an increasingly common disease, characterized by stomach acid reflux reaching the upper airways. Postnasal drip (PND) is a known consequence of LPR, defined as mucus accumulation perceived in the posterior areas of the nose and throat. PND is among the most common causes of persistent cough, hoarseness, sore throat, and other symptoms, affecting the quality of life. This study aimed to evaluate the effects of a proton-pump inhibitor (PPI) on PND symptoms in patients with LPR.
Methods
We prospectively enrolled patients diagnosed with LPR at our institution between September 2019 and June 2020. The patients were randomly assigned to either the treatment group (20 mg of ilaprazole daily for 8 weeks) or the control group. The scores for the Reflux Symptom Index (RSI), Reflux Finding Score (RFS), and Sino-Nasal Outcome Test (SNOT)-20 were evaluated at baseline and at the end of treatment, focusing on PND symptoms.
Results
Eighty patients (28 men and 52 women; mean age, 48.8 years, range, 22–78 years) were enrolled, with 43 in the treatment group and 37 in the control group. The initial RSI, RFS, and SNOT-20 scores were similar between the two groups, and they decreased significantly only in the treatment group (p=0.002, p<0.001, and p=0.015, respectively). However, the PND symptom scores showed a significant decrease in the treatment group only in the RSI (p=0.012).
Conclusion
PPI treatment for 8 weeks may be effective in improving PND symptoms in patients with LPR.
INTRODUCTION
Laryngopharyngeal reflux (LPR) is a condition that occurs when stomach acids and contents reflux into the upper respiratory and digestive tracts, areas with relatively weak defense mechanisms. This condition is marked by a variety of symptoms, including frequent throat clearing, a hoarse voice, the sensation of a foreign object in the throat, a sore throat, chronic cough, and postnasal drip (PND) [1]. LPR is highly prevalent, as illustrated by reports finding that 10%–20% of patients visiting an otolaryngology outpatient clinic were diagnosed with LPR, and more than 50% of patients complaining of a hoarse voice had LPR [2-4].
PND, which is one of the main issues of LPR, causes discomfort and continuous throat clearing, as excessive mucus from the nasal cavity flows through the pharynx, and significantly diminishes patients’ quality of life. In clinical practice, otolaryngologists frequently encounter patients with LPR who complain of PND and cough-induced discomfort.
The purpose of this study was to evaluate the efficacy of medical therapy with a proton-pump inhibitor (PPI) to reduce LPR-induced PND symptoms and improve patients’ quality of life, assessed using subjective questionnaire surveys.
METHODS
This study was designed as a prospective randomized controlled trial. Between September 2019 and June 2020, patients who visited the Department of Otorhinolaryngology at Chungbuk National University Hospital complaining of symptoms related to LPR underwent laryngeal endoscopy and completed a reflux symptom questionnaire. Patients diagnosed with LPR were enrolled in the study. The inclusion criteria were age over 18 years, no history of smoking or professional voice use, and high compliance with lifestyle and dietary changes over the 8-week study period. Patients with allergic rhinitis or sinusitis, asthma, cystic fibrosis, or severe systemic diseases were excluded, as were those who had used medication for LPR in the month before their visit. This study was approved by the Clinical Trial Ethics Committee of Chungbuk National University Hospital (IRB no. 2019-05-015), and all patients provided written informed consent.
LPR was diagnosed based on several symptoms, such as frequent throat clearing, hoarseness, throat irritation, and chronic cough, lasting for more than 3 months, as well as the Reflux Symptom Index (RSI) score and the Reflux Finding Score (RFS) obtained during laryngoscopy [5-7]. The RSI [7] considers 9 symptoms rated on a scale from 0 to 5, according to their impact on daily life (0=no problem, 5=severe problem), and was used to quantify the severity of the reflux symptoms. The RFS [6] evaluates 8 items (subglottic edema, ventricular obliteration, erythema/hyperemia, vocal fold edema, diffuse laryngeal edema, posterior commissure hypertrophy, granuloma/granulation tissue, and thick endolaryngeal mucus), on a scale from 0 to 26 based on laryngoscopy findings. Patients with suspected LPR lasting for more than 3 months and with an RSI score ≥13 or an RFS score ≥7 were diagnosed with LPR. Furthermore, the Sino-Nasal Outcome Test (SNOT)-20 questionnaire was used to measure the quality of life in relation to the nasal symptoms. This tool scores rhinologic and ear/facial symptoms, sleep disorders, and psychological dysfunction, on a scale from 0 to 5 for each group, with a total score of 20 [8].
The participants were randomly assigned to either the medication or the control group, based on their medical record numbers. Both groups received education on dietary habits, and the medication group was administered oral ilaprazole (20 mg, once daily) for 8 weeks. After completing the treatment, the RSI, SNOT-20, and RFS were re-evaluated. The RFS was assessed through laryngoscopy performed by the same examiner, blinded to the assigned group and previous results.
Statistical analyses were performed using the independent-samples t-test to compare two groups, and the paired t-test to compare symptoms before and after the treatment period within each group. The analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), and p-values <0.05 were considered statistically significant.
RESULTS
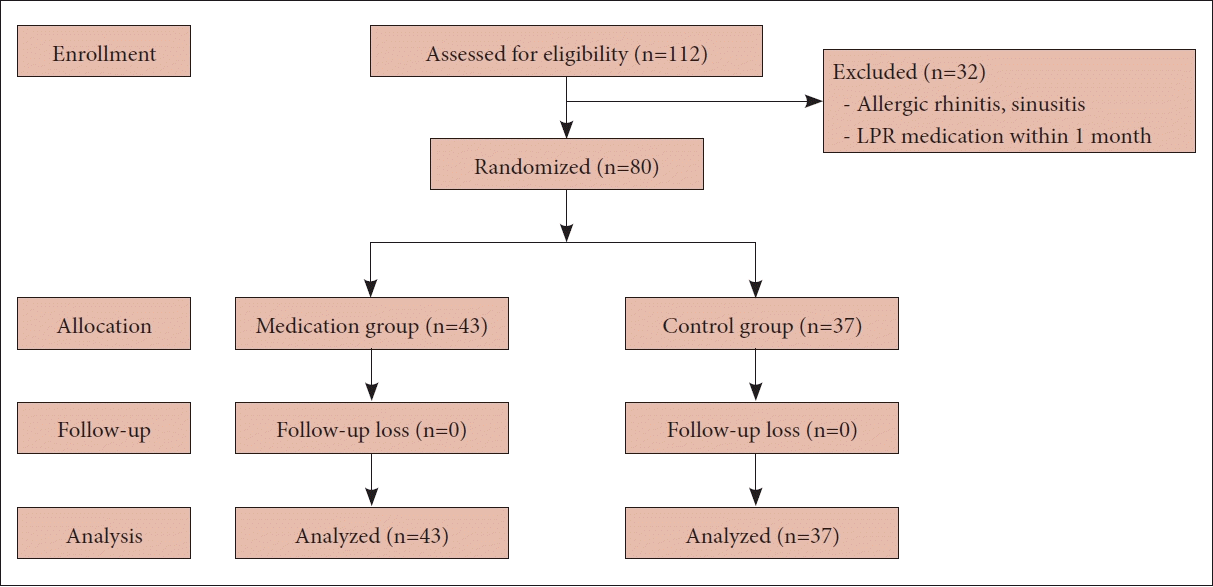
The study included 80 patients (52 women and 28 men), with a mean age of 48.8 years (range, 22–78 years). Participants were randomly assigned to either the medication group (43 patients) or the control group (37 patients), as depicted in Fig. 1. There was no significant difference in the distribution of age and sex between the two groups, as shown in Table 1.
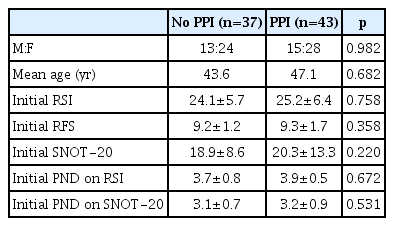
The mean RSI score in all patients before the start of the study was 24.5±5.4 (range: 14–39). The highest score among individual symptoms was recorded for “lump in the throat,” with a mean of 4.2, followed by “frequent throat clearing,” “postnasal drip,” and “cough.” The mean RFS score was 9.2±1.9 (range: 3–14), with vocal fold edema scored at a mean of 1.3 points and erythema/hyperemia at 2.9 points, and the mean SNOT-20 score was 19.3±8.2 (range: 5–47). The mean PND score was 3.9±1.1 on the RSI and 3.1±1.4 on the SNOT-20. No significant differences were noted between the medication and control groups before the start of the study (Table 1).
After 2 months of treatment, the RSI score decreased from 25.2±6.4 to 15.3±7.1 in the medication group (p=0.002), whereas in the control group it decreased from 24.1±5.7 to 17.2±3.4, although the difference was not statistically significant (p=0.084) (Fig. 2A). The RFS score decreased from 9.3±1.7 to 4.2±1.1 in the medication group (p<0.001) and from 9.2±1.2 to 4.4±2.2 in the control group (p=0.039) (Fig. 2B). The SNOT-20 score decreased from 20.3±13.3 to 15.6±17.9 in the medication group (p=0.015) and from 18.9±8.6 to 17.9±7.6 in the control group (p=0.271) (Fig. 2C).
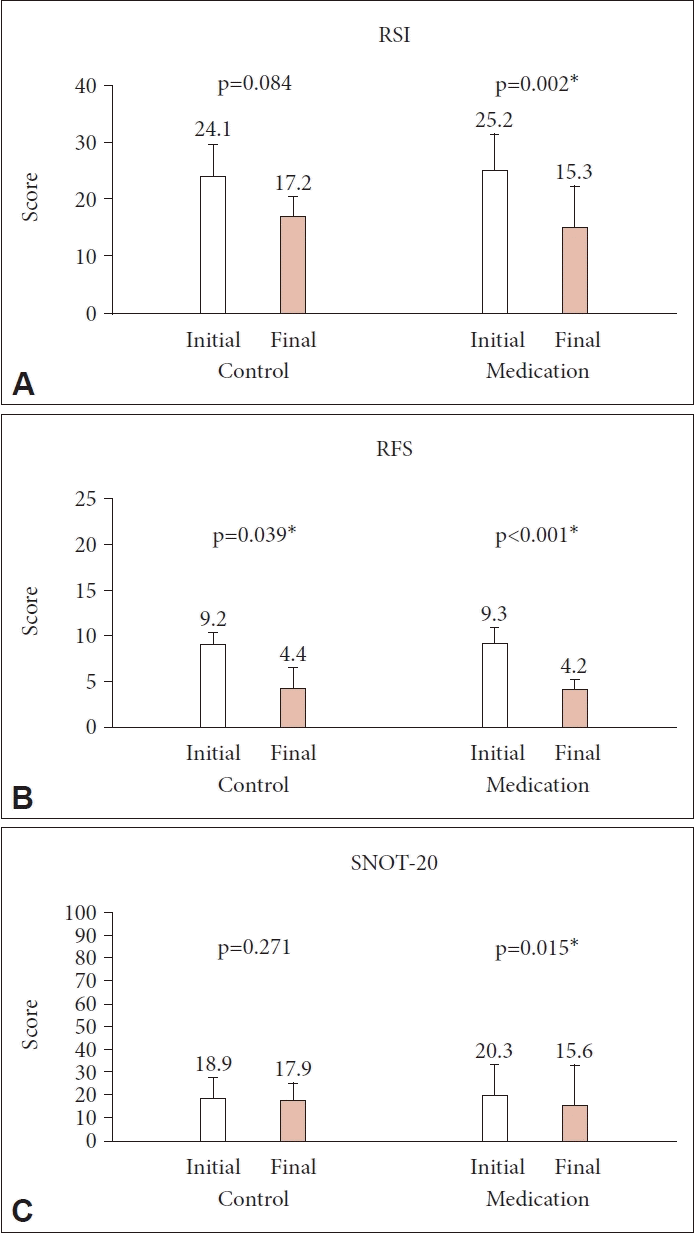
Initial and final RSI (A), RFS (B), and SNOT-20 (C) scores in the medication and control groups (*p<0.05). RSI, Reflux Symptom Index; RFS, Reflux Finding Score; SNOT-20, Sino-Nasal Outcome Test-20.
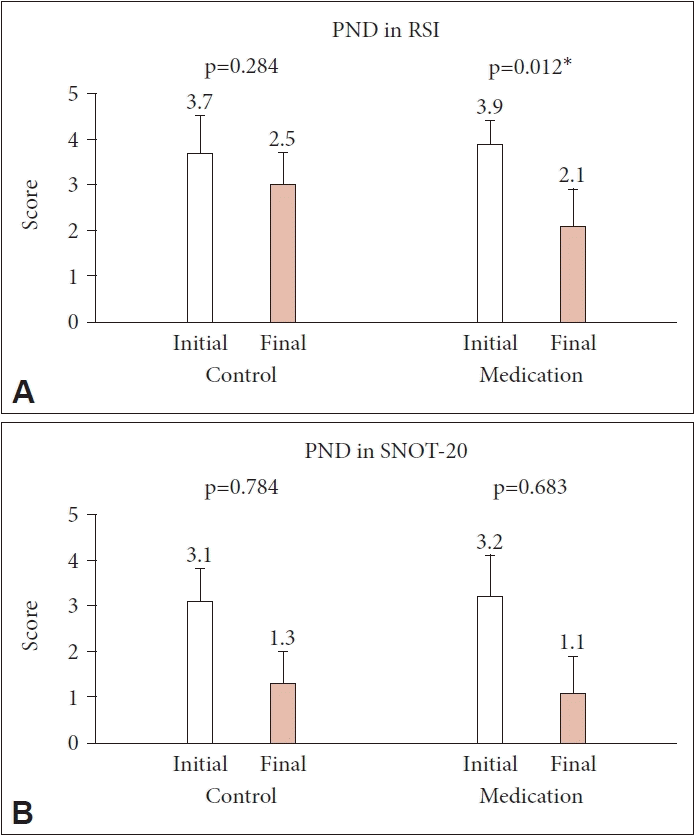
The PND score in the RSI decreased from 3.9±0.5 to 2.1±0.8 in the medication group (p=0.012), whereas in the control group it decreased from 3.7±0.8 to 2.5±1.2 (p=0.284) (Fig. 3A). The PND score in the SNOT-20 also decreased from 3.2±0.9 to 1.1±0.8 in the medication group and from 3.1±0.7 to 1.3±0.7 in the control group, with no statistically significant differences (p=0.683 and p=0.784, respectively) (Fig. 3B).
DISCUSSION
LPR is a common diagnosis in the field of otolaryngology. However, it is often overlooked as a sign of neurological disorders in daily life, resulting in incorrect treatment. PND can also cause discomfort to patients, with mucus observed in the nasopharynx and oropharynx, even in the absence of a clear respiratory disease. This condition often proves challenging to manage solely within the field of otolaryngology, resulting in decreased patient satisfaction with treatment [9]; moreover, PND is one of the most common causes of chronic cough [10].
Although there are various causes of PND, including rhinitis and chronic sinusitis, LPR is also an important cause of PND [11,12]. Although PND is included in the RSI and has been assessed in the context of other LPR symptoms, relatively few previous studies have evaluated the efficacy of PPI therapy on PND symptoms. A previous study reported that more than 50% of subjects with PND had positive pharyngeal pH probes [13]. Koufman et al. [14] observed a similar incidence of abnormal pH probe results in patients with PND symptoms. In addition, Wise et al. [15] reported objective evidence of LPR in patients with PND; therefore, reflux treatment may reduce PND complaints. In this study, the use of a PPI significantly reduced the severity of PND symptoms, as evaluated using questionnaires and the SNOT-20 scale. This finding suggests that the empirical use of PPIs is appropriate for patients complaining of PND symptoms, as LPR may be suspected as the cause.
This study has some limitations; namely, the placebo effect was not evaluated, and both groups received dietary education. In addition, the LPR diagnosis was based on clinical symptoms and RSI and RFS scores, and objective 24-hour dual-probe pH monitoring tests were not performed [16]. However, the RSI and RFS are recognized as effective alternative methods for the diagnosis and follow-up of LPR, compared to the invasive 24-hour dual-probe pH monitoring tests [17-19].
In conclusion, PPI therapy provided significant improvement in PND symptoms in patients with LPR. Therefore, clinicians should evaluate the possible presence of LPR in patients who report PND symptoms and consider the empirical use of PPIs to ameliorate the conditions of these patients.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Hahn Jin Jung. Data curation: Min Jai Cho. Formal analysis: Min Jai Cho. Investigation: Seung Heon Kang. Methodology: Hahn Jin Jung. Supervision: Hahn Jin Jung. Visualization: Jun Yong Lee, Seung Heon Kang. Writing—original draft: Jun Yong Lee. Writing—review & editing: Hahn Jin Jung.
Funding Statement
None