INTRODUCTION
Heat shock protein 70 (Hsp70), a molecular chaperone, participates in protein folding and the prevention of protein aggregation in the cytoplasm [
1]. The presence of extracellular Hsp70 and its role as a damage-associated molecular pattern in various organs, including airways, have been reported [
2,
3]. Increased levels of extracellular Hsp70 have been detected in the serum of patients with asthma and in the bronchoalveolar lavage fluid of mice with allergic rhinitis (AR). Hsp70 affects the antigen activity of innate immune cells, and the chaperone activity of Hsp70 is involved in the pathogenesis of allergic airway disease through the regulation of unfolded protein responses induced by endoplasmic reticulum stress [
2].
The chemokine receptor CXCR4 induces cellular migration, hematopoiesis, and cell homing. Neutrophils highly expressing CXCR4 are essential for the pathogenesis of the allergic airway response triggered by environmental factors [
4]. Additionally, CXCR4 is involved in the pathogenesis of AR by interacting with its ligand, CXCL12 [
5,
6].
Lipopolysaccharides (LPS) are one of the most common pathogen-associated molecular patterns, and several LPS molecules can be integrated into receptor complexes to function as pattern recognition receptors. Hsp70 and CXCR4 have been identified as components of this receptor complex, suggesting their essential roles in initiating LPS-induced innate immune responses [
7]. Additionally, Hsc/Hsp70-interacting protein (Hip) directly binds to CXCR4 and participates in CXCR4 internalization, which is important in the activation of mechanisms downstream of the CXCR4 pathway [
8].
Since Hsp70 and CXCR4 constitute a common receptor complex, Hip directly interacts with CXCR4, and both extracellular Hsp70 and CXCR4 are important mediators in allergic airway diseases, we hypothesized that extracellular Hsp70 may be involved in the CXCR4-dependent signaling pathway. In this study, we primarily aimed to evaluate the relationship between extracellular Hsp70 and CXCR4 and their role in the primary human nasal epithelium.
METHODS
Experimental procedures using human nasal epithelial (HNE) cells were approved by the Institutional Review Board of Chung-Ang University Hospital (2020-002-405). Primary HNE cells were cultured in an air–liquid interface (ALI) environment as previously described [
3]. Primary HNE cells from day 14 of the ALI culture were used in the experiments. Hsp70 (100 ng/mL; BD Biosciences, East Rutherford, NJ, USA) was added to the apical and basolateral culture media and maintained for the indicated durations. For single-cell quantitative polymerase chain reaction (PCR), RNA was extracted from two independent primary HNE cultures using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RNasefree DNase (Qiagen, Hilden, Germany) was added to the extracts in accordance with the manufacturer’s instructions. The extracted RNA samples were sequenced by Macrogen (Seoul, Republic of Korea; HiSeq 4000 instrument, Illumina Inc., San Diego, CA, USA), and sequencing results were analyzed. The raw reads from the sequencer were processed to remove lowquality and adapter sequences before analysis, and the processed reads were aligned to
Homo sapiens (hg19) using HISAT v2.0.5 (
https://daehwankimlab.github.io/hisat2/). Western blot analysis was performed using an anti-CXCR4 antibody, an anti-GAPDH antibody, and antibodies against the components of mitogen-activated protein kinase (MAPK) pathways (Cell Signaling Technology, Danvers, MA, USA) as previously described [
3]. CXCL12 (250 ng/mL; R&D Systems, Minneapolis, MN, USA) was applied to the apical and basal culture media to activate the CXCL12/CXCR4 signaling pathway [
9,
10]. An anti-Hsp70 antibody (2 μg/mL; Cell Signaling Technology) and AMD3100 (6 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) were used to inhibit the functions of extracellular Hsp70 and CXCR4, respectively. Western blotting was repeated at least thrice in independently cultured primary HNE cells.
Data were expressed as mean±standard deviation. Fold differences between groups were compared via the Mann–Whitney U test. Statistical significance was set at p<0.05. Data were statistically analyzed in GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Primary HNE cells were incubated with Hsp70 for 24 h, and single-cell RNA sequences were analyzed. Hsp70 treatment significantly changed the expression of various genes in primary HNE cells compared to the control (
Table 1). Among them,
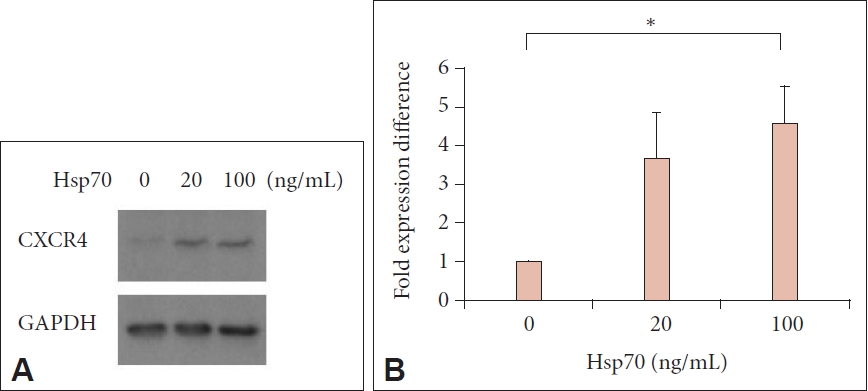
CXCR4 was the only target gene, whose expression was upregulated more than 3-fold by Hsp70 treatment, and it has been reported to be involved in the pathogenesis of allergic inflammation. Western blot analysis indicated that CXCR4 expression significantly increased after Hsp70 treatment (p<0.05) (
Fig. 1).
We evaluated whether extracellular Hsp70 affected the downstream signaling pathway of CXCR4. We treated cells with Hsp70 with or without CXCL12, a well-identified CXCR4 agonist, and evaluated the downstream MAPK pathways via western blotting. We found that the expression of phospho-ERK was increased by cotreatment with Hsp70 and CXCL12 but inhibited by pretreatment with AMD3100, a CXCR4 inhibitor (
Fig. 2A). Furthermore, pretreatment with the anti-Hsp70 antibody reduced the upregulation of phospho-ERK expression induced by cotreatment with Hsp70 and CXCL12 (
Fig. 2B).
DISCUSSION
In this study, the extracellular administration of Hsp70 upregulated CXCR4 expression at gene and protein levels. Extracellular Hsp70 enhanced the downstream signaling pathway after CXCL12/CXCR4 binding, as demonstrated by increased phospho-ERK expression. These findings suggested that extracellular Hsp70 might be involved in the CXCL12/CXCR4 signal transduction pathway in primary HNE cells.
The pro-inflammatory and anti-inflammatory activities of Hsp70 have been identified in the upper airway. Hsp70 treatment prevents airway eosinophilia and allergen-induced cytokine release in mice with allergic asthma [
11]. It also induces the production of interleukin (IL)-8 and tumor necrosis factor-α in human airway epithelial cells [
12]. Although extracellular Hsp70 may be involved in the pathogenesis of AR [
3], the underlying mechanisms are largely unexplored.
Previous studies demonstrated that the CXCL12/CXCR4 pathway plays a pivotal role in airway inflammation and airway hyperresponsiveness [
5,
6]. CXCR4 expression in the airway increases during the development of allergic airway disease, and CXCR4 inhibition reduces airway eosinophilia [
6]. Furthermore, the inhibition of CXCL12/CXCR4 interaction decreases the level of Th2-associated cytokines, such as IL-4 and IL-5 [
5].
On the basis of the current and past reports, we suggested that extracellular Hsp70 might participate in the CXCL12/CXCR4 signaling pathway in primary HNE cells. To the best of the authors’ knowledge, this study is the first to evaluate the relationship between Hsp70 and the CXCR4 pathway in primary HNE cells. Given that extracellular Hsp70 and the CXCR4 pathway are important for the pathogenesis of AR, our preliminary study implies that future studies should focus on the interaction of Hsp70 and the CXCR4 pathway and its role in the development of upper airway diseases such as AR.
CXCR7 is a newly discovered receptor for CXCL12, and it has been found to be highly expressed in many tumor cells [
13]. In a previous study, CXCR7 was reported to be expressed in lower airway epithelial cells, and CXCR7 played a role in regulating allergic airway inflammation in a mouse experiment [
14]. In our study, we did not find a significant change in genetic expression level of CXCR7 (data not shown). Future research on CXCR7 might be helpful in understanding the role of CXC12-mediated allergic airway inflammation.
There are several limitations to our study. First, our study was based on in vitro data using primary NHE cells. Therefore, in vivo experimental data, such as using an AR mouse model or human nasal tissues with or without the inhibition of extracellular Hsp70, are needed. Second, we did not further evaluate the downstream signaling pathway after CXCL12/CXCR4 axis activation. Further components of the downstream signaling pathway, such as NF-κB, need to be evaluated.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print