|
 |
| J Rhinol > Volume 30(2); 2023 |
|
Abstract
The last decade has seen the emergence of immune checkpoint inhibitors for the treatment of a wide variety of cancer types. While these medications are generally speaking well tolerated, the full long-term side effect profiles of these medications have not been fully elucidated. We describe a case of chronic rhinosinusitis with nasal polyposis induced treatment with pembrolizumab, the first reported case. We present the case of a 48-year-old man with a background history of stage IV non-small cell lung cancer with bone metastases. He was commenced on pembrolizumab and over the course of the subsequent 5 years he developed significant nasal polyps bilaterally, and was commenced on medical therapy. Sinus CT scan demonstrated bilateral total opacification of all his sinuses and nasal cavity. He subsequently underwent bilateral functional endoscopic sinus surgery. He remains symptom-free and at his last clinical follow-up visit 1 year later. There are limited case reports of nasal polyposis occurring in patients receiving immune checkpoint inhibitors, with only one case requiring surgery. We describe the first case of severe nasal polyps due to pembrolizumab and successfully treated with polypectomy. From our review, there were no cases that required a cessation of therapy.
The last decade has seen the emergence of immune checkpoint inhibitors (ICIs) for the treatment of a wide variety of cancer types. The first licensed ICI was ipilimumab in 2011, targeting cytotoxic T-lymphocyte associated protein 4 (CTLA4), followed by pembrolizumab and nivolumab, targeting programmed cell death 1 (PD-1). These monoclonal antibodies are now being used as either single or combination treatment in over 50 different cancer types, and there are currently in excess of 3,000 active clinical trials evaluating these drug treatments, constituting about two thirds of all oncology trials [1]. ICIs work by activating the host immune system’s anti-tumor mechanisms.
While these medications have no doubt revolutionized the field of oncology and are generally speaking well tolerated, the full long-term side effect profiles of these medications have not been fully elucidated.
To that end, we describe a case of chronic rhinosinusitis (CRS) with nasal polyposis induced by several years of treatment with pembrolizumab, the first reported case in the literature.
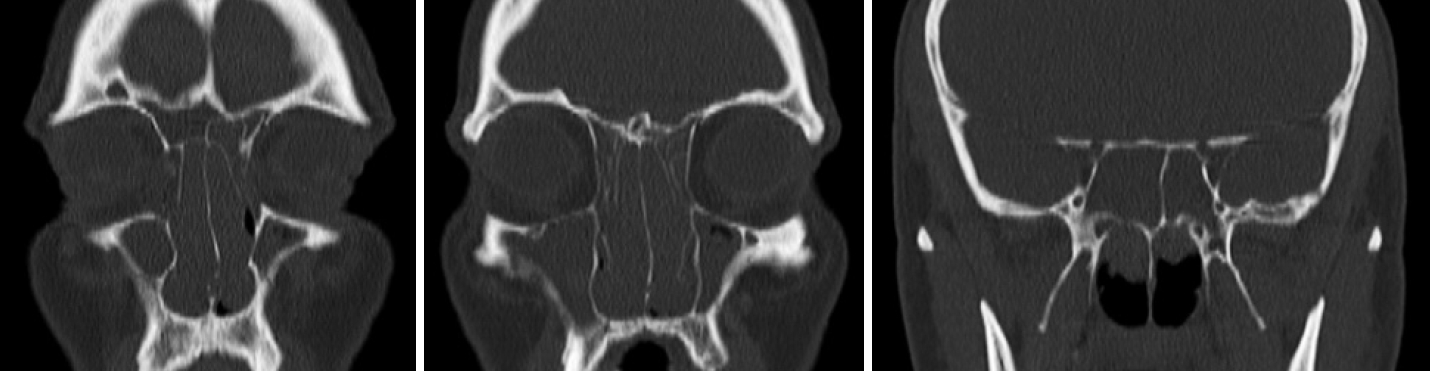
We present the case of a 48-year-old man with a background history of stage IV non-small cell lung cancer (NSCLC) with bone metastases. He was commenced on pembrolizumab as part of a clinical trial in 2015. Over the course of the subsequent 5 years, he developed progressive severe bilateral nasal blockage, anterior rhinorrhoea, post-nasal drip, and hyposmia, which subsequently prompted referral to the otolaryngology service. On examination, he was found to have significant grade 3 nasal polyps bilaterally and was commenced on medical therapy, with a two-week course of topical betamethasone sodium phosphate nasal drops followed by combination of azelastine hydrochloride and fluticasone propionate nasal spray. He had mild improvement in his symptoms. Sinus CT scan demonstrated bilateral total opacification of all his sinuses and near total obstruction of the nasal cavity (Fig. 1). Notably, prior to commencing treatment with pembrolizumab, he did not have any evidence of sinus disease on pretreatment radiological investigations and was not suffering from any sinus symptoms (Fig. 2).
He subsequently underwent bilateral functional endoscopic sinus surgery (FESS) 5 years after commencing pembrolizumab, in the form of a “full house FESS”: nasal polypectomy, bilateral uncinectomy, middle meatal antrostomy, anterior and posterior ethmoidectomy, frontal pathway clearance, and wide sphenoidotomy. He was discharged home well, on day 1 postoperatively, on maximal medical therapy, topical budesonide nasules for 4 weeks, followed by maintenance with combination of azelastine hydrochloride, fluticasone propionate nasal spray, and sodium chloride nasal rinse. Final histological analysis revealed inflammatory polyps and preoperative investigations did not reveal any evidence of eosinophilia. He remains symptom-free and at his last clinical follow-up visit 1 year after his surgery he had no evidence of recurrence of his nasal polyps endoscopically or radiologically. He remains on pembrolizumab for his NSCLC and topical therapy for his nasal polyposis and continues to undergo regular clinical and radiological surveillance for his NSCLC.
Pembrolizumab is a highly selective monoclonal antibody against PD-1, a negative stimulatory receptor expressed on the surface of activated T-cells [2]. Side effects of ICIs, such as pembrolizumab, vary greatly. The most commonly reported side effects include fatigue (19.4%) and pruritis (10.7%) [2]; however, severe immune-related adverse effects (irAEs), such as colitis, hepatitis, endocrinopathies, and pneumonitis, have also been reported [3]. irAEs occur due to immune activation and it has been theorized that their occurrence may signal a better response to these medications [4].
To date, there have been no reported cases of pembrolizumab causing CRS with or without nasal polyposis. One previous case has been described of worsening of allergic fungal CRS in the setting of pembrolizumab therapy [5], and there have been reports of nivolumab, another ICI of programmed deathligand 1 (PDL-1), causing CRS with nasal polyposis [5,6]. In one case this occurred years after commencing therapy [6]. Another case occurred, however without reported polyposis, in a patient following their third infusion, and CT at that time showed maxillary and ethmoid sinusitis [7]. In the same paper, investigators reported a patient commenced on ipilimumab for malignant melanoma who developed symptoms of sinusitis 1 year into therapy, and a CT scan demonstrated pansinusitis. Neither patient responded to antibiotic therapy but both improved with anti-tumour necrosis factor (TNF) therapy [7].
Watanabe et al. [8] discussed a case of a 71-year-old female commenced on combination of nivolumab and ipilimumab for stage IV renal cell carcinoma. The patient developed eosinophilic airway inflammation and CRS after two cycles of therapy. She was found to have nasal polyposis and sinusitis. Polypectomy was performed and histological exam showed dense infiltration with eosinophils. However, the authors suggested that it is unlikely that the polyps developed in such a short timeframe but were likely aggravated by the checkpoint inhibitors [8].
Recently, several new biologic agents targeting various pathways of the immune system have been used to treat CRS with nasal polyposis, including dupilumab targeting interleukin (IL)-4/IL-13, omalizumab targeting Ig-E, and mepolizumab and benralizumab targeting IL-5. These have shown promise in treating severe cases of eosinophilic CRS with nasal polyposis, and provide some insight into the immunological mechanisms that underlie this pathological process [9].
Studies on irAEs of ICIs have identified higher levels of IL-6 after commencement of treatment, which was closely related to the development of irAEs. Other inflammatory cytokines, such as CXCL9/10/11/13, were also found to be upregulated following immune checkpoint inhibition; however, there is limited evidence of their effects on IL-4/5 and Ig-E, the main immunological mediators of CRS with nasal polyposis [10]. Interestingly, ICI-induced eosinophilic inflammation of the lungs has been described with various manifestations [11,12].
Finally, T-cell infiltration has been suggested as a potential mechanism behind irAEs on other organs, and T-cell infiltration has been described in one case of ICI-related deterioration of allergic CRS [5]. Underlying T-cell related inflammatory changes are possible mechanisms for ICI-related CRS, but secondary infections may still occur [5].
There are limited case reports of CRS occurring in patients receiving ICIs, with only one case requiring surgery. We describe the first case of severe CRS with nasal polyps due to pembrolizumab and successfully treated with polypectomy. From our review, there were no cases that required a cessation of therapy. Further research is required to evaluate CRS with or without nasal polyposis as an irAE of immune checkpoint inhibition. Patients on ICIs should be screened for sinonasal side effects.
Notes
Ethics Statement
Ethical approval was granted by the Beaumont Hospital Institutional Review Board, as well as by the patient.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Author Contributions
Conceptualization: all authors. Data curation: all authors. Formal analysis: all authors. Investigation: all authors. Methodology: all authors. Project administration: all authors. Resources: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
References
1) Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nat Rev Drug Discov 2019;18(12):899–900.



2) Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372(21):2018–28.


3) Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2(10):1346–53.


4) Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18(1):87.




5) Krane NA, Beswick DM, Sauer D, Detwiller K, Shindo M. Allergic fungal sinusitis imitating an aggressive skull base lesion in the setting of pembrolizumab immunotherapy. Ann Otol Rhinol Laryngol 2021;130(1):108–11.



6) Kassem F, Rosman Y, Blau I, Nageris B, Zakharov A, Biadsee A. Nivolumab-induced diffuse type 2 rhinosinusitis: a case report. Asian Pac J Allergy Immunol 2021 Dec 26 [Epub]. Available from: https://doi.org/10.12932/AP-240721-1196.

7) Dein E, Sharfman W, Kim J, Gellad F, Shah AA, Bingham CO 3rd, et al. Two cases of sinusitis induced by immune checkpoint inhibition. J Immunother 2017;40(8):312–4.



8) Watanabe H, Asada K, Shirai T, Torii H, Yoshimura K, Kusafuka K. Eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis during nivolumab and ipilimumab. Respirol Case Rep 2020;8(7):e00638.




9) Patel GB, Kern RC, Bernstein JA, Hae-Sim P, Peters AT. Current and future treatments of rhinitis and sinusitis. J Allergy Clin Immunol Pract 2020;8(5):1522–31.



10) Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ, Yao Y, et al. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res 2020;39(1):284.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print