Prognostic Factors for Survival or Severity After COVID-19 Infection in Cancer Patients: A Systematic Review and Meta-Analysis
Article information
Abstract
Background and Objectives
Cancer organizations worldwide have recently released care guidelines for cancer patients with coronavirus disease 2019 (COVID-19). Several studies have reported higher mortality rates in cancer patients with COVID-19. However, drawing robust conclusions remains challenging due to a lack of research on clinical prognostic factors in this patient group.
Methods
A comprehensive literature search was conducted using the PubMed, Embase, and Cochrane databases. We searched the keywords in the following combination: (“COVID-19” or “coronavirus” or “wuhan virus”) and (“cancer”). The search was performed on August 1, 2020, and only papers written in English were included in this study. We collected data from 3,215 cancer patients with COVID-19 from 16 studies and analyzed overall mortality after COVID-19 infection in cancer patients compared to controls, as well as prognostic factors for severity and mortality after COVID-19 infection. The prognostic factors analyzed encompassed demographics, comorbidities, symptoms, cancer treatment within 4 weeks of COVID-19 diagnosis, and treatment for COVID-19 infection.
Results
This meta-analysis evaluated mortality rates and related prognostic factors in cancer patients infected with COVID-19. First, 15 of the 16 studies reported mortality data; 663 patients died among a total of 3,215 people, resulting in a combined mortality rate of 21%. Second, the following poor prognostic factors were identified: male sex, older age (≥65 years), respiratory symptoms (e.g., cough and dyspnea), and other comorbidities (e.g., cardiovascular disease, hypertension, and chronic obstructive pulmonary disease).
Conclusion
The mortality of cancer patients infected with COVID-19 can reach about 20%.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This highly transmissible and pathogenic virus, with no true precedent in modern times, has been responsible for a rapidly evolving crisis worldwide that has impacted every aspect of life. Similar to infections caused by other respiratory viruses, elderly or immunocompromised individuals with underlying health conditions, such as diabetes, lung disease, cancer, immunodeficiency, heart disease, hypertension, asthma, kidney disease, gastrointestinal/liver disease, and obesity, are more susceptible to SARS-CoV-2 [1].
In particular, cancer patients have been disproportionately affected by the COVID-19 pandemic, both directly and indirectly [2]. Therefore, these patients have experienced adverse outcomes related to COVID-19. Numerous studies have reported that patients with comorbidities, such as cancer, appear to be at a higher risk for a complicated course, leading to increased mortality [3-5]. To date, only a few reports have compared data regarding COVID-19 patients with and without cancer, and the prognostic factors affecting these patients have not been studied. Currently, several multi-institutional initiatives are working to aggregate data on COVID-19 patients with cancer, including the COVID-19 and Cancer Consortium (CCC19), Thoracic Cancers International COVID-19 Collaboration (TERAVOLT), the UK Coronavirus Cancer Monitoring Project, and others [6-8]. This global trend underscores the significance of COVID-19 infections in cancer patients today.
There is limited published literature on cancer patients with COVID-19 infections, and the available studies exhibit significant heterogeneity. In order to better assess the impact of COVID-19 on cancer patients, we collected data from 16 studies involving 3,215 infected individuals and conducted a meta-analysis. The primary objective of this meta-analysis was to compare mortality rates between COVID-19 patients with and without cancer. The secondary objective was to evaluate various prognostic factors influencing the severity of COVID-19 in cancer patients.
METHODS
Literature sources and study identification
We conducted this systematic review and meta-analysis in accordance with the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) statement. Our literature search spanned the PubMed, Embase, and Cochrane databases, using the following keyword combinations: (“COVID-19” or “coronavirus” or “wuhan virus”) and (“cancer”). We searched for relevant literature on August 1, 2020, starting from the onset of COVID-19 in December 2019. Two independent reviewers screened all abstracts and titles for potential studies, excluding those deemed irrelevant. Following the initial screening, the full manuscripts were reviewed for eligibility, with any disagreements resolved through consensus.us.
Exclusion, inclusion criteria and data abstraction
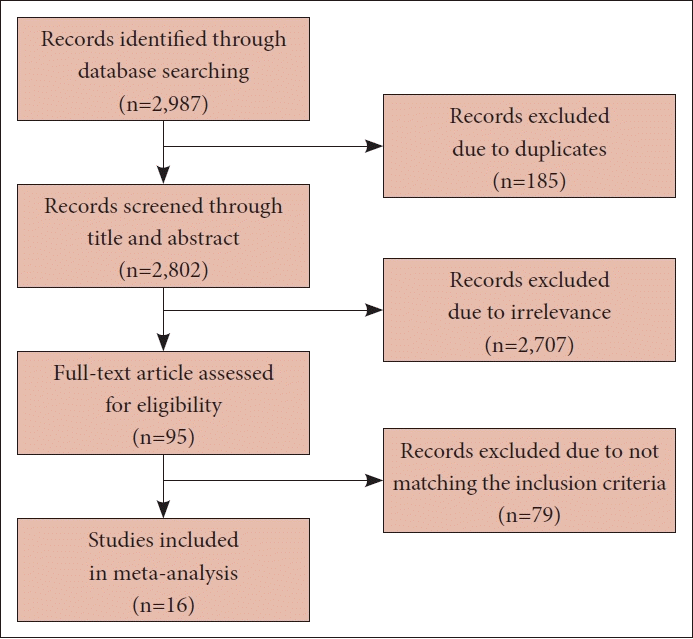
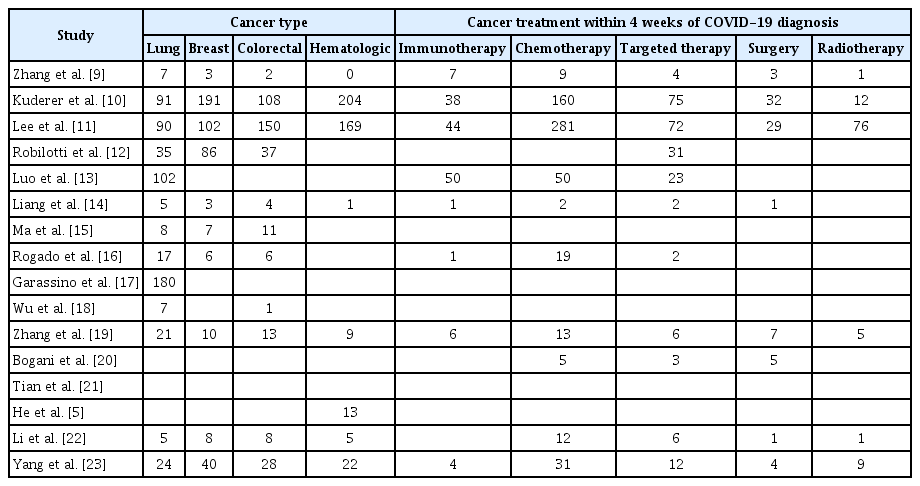
A total of 2,987 relevant studies were identified through a literature search. Of these, 185 studies were excluded due to duplication. Upon reviewing the abstracts, an additional 2,707 studies were excluded for the following reasons: 1) they were written in languages other than English, 2) they were review articles, or 3) they did not provide information about the prognosis of cancer patients. We then reviewed the full text of the remaining 95 studies, and only 16 studies met the inclusion criteria [5,9-23]. However, two studies by Rogado et al. were based on the same patient group at the same hospital, so we included only one of these studies [16].
The primary study inclusion criteria were as follows: 1) published as original articles; 2) evaluated cancer patients infected with COVID-19; 3) (a) compared living and deceased patients, (b) compared mild and severe (including deceased) patients, (c) provided information on individual patients; and 4) presented odds ratios (OR) with 95% confidence intervals (95% CI) or 2×2 tables for various prognostic factors. Data were collected on the first author, country, total number of patients, number of deceased and male patients, median age, tumor site and stage, types of recent cancer treatments, comorbidities, symptoms, and treatment of COVID-19 infection.
Statistical analyses
To evaluate the overall prognosis of cancer patients, we compared cases with mild outcomes (alive) and severe outcomes (defined according to the criteria used in each paper). A further analysis was conducted, focusing on studies that reported only whether patients survived or died, to assess the prognostic factors for mortality alone. We collected ORs and 95% CIs if they were reported. If a direct report of the OR was not available, the OR and 95% CI were calculated based on a 2×2 table using OR calculator software (Select Statistical Services, Exeter, UK; https://select-statistics.co.uk/calculators/confidence-interval-calculator-odds-ratio/). The OR and its corresponding 95% CI were combined from each study using statistical software (Comprehensive Meta-Analysis Version 2.0, Biostat, Englewood, NJ, USA). Only a DerSimonian-Laird random-effects model was used, regardless of the degree of heterogeneity, because we believed that the studies were inherently heterogeneous. Nonetheless, the p-values for the Cochran Q statistic test and I2 values are presented in Supplementary Table 1 (in the online-only Data Supplement). A subgroup analysis was conducted of studies that only reported mortality as an outcome to evaluate the prognostic factors for mortality. To detect publication bias, funnel plots, the Begg test, and the Egger test were used. The trim and fill method was also employed to estimate the effect size after correcting for publication bias. All p-values were two-tailed. The MOOSE checklist was utilized for quality control of this meta-analysis, which was conducted using observational studies (https://www.elsevier.com/__data/promis_misc/ISSM_MOOSE_Checklist.pdf).
RESULTS
A flow chart depicting the selection process for the included studies can be seen in Fig. 1.
All seven Chinese studies were conducted in Wuhan [9,14,15,18,19,21], and several hospitals were repeatedly included in other studies, which may have led to a significant number of duplicated patients. However, accurate differentiation was not possible; thus, all patients were included in the meta-analysis. All included studies are summarized in Tables 1 and 2. From these studies, data from 3,233 cancer patients infected with COVID-19 were screened and used in the meta-analysis. Detailed information regarding heterogeneity and publication bias is provided in Supplementary Table 1 and Supplementary Fig. 1 (in the online-only Data Supplement).
Mortality after COVID-19 infection in cancer patients
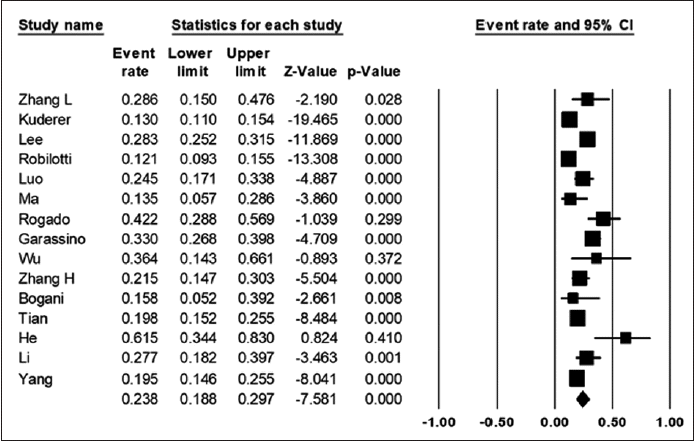
Fifteen out of the 16 studies reported a death count. Among a total of 3,215 cancer patients with COVID-19, 663 died. The combined mortality rate was 23.8% (95% CI, 18.8%–29.7%). However, this outcome is believed to have been influenced by publication bias, and the adjusted result is 21% (95% CI, 19%–27%) (Fig. 2).
Prognostic factors for mortality after COVID-19 infection in cancer patients
Sex, age, and metastasis
Men exhibited a worse prognosis than women. The likelihood of men developing a severe case was 1.75 (95% CI, 1.36–2.27) times higher than that of women. In individuals aged 65 years or older, the probability of a severe case increased by 1.93 (95% CI: 1.41–2.66) times. However, this result was suspected to be influenced by publication bias, and the corrected OR was 1.80 (95% CI, 1.37–2.37). Metastasis did not impact the prognosis. The specific values of the ORs and 95% CIs can be found in Fig. 3.
Comorbidities
Cardiovascular disease (OR=2.00 for overall prognosis, OR=2.17 for mortality), hypertension (OR=1.67 for overall prognosis, OR=1.63 for mortality), and chronic obstructive pulmonary disease (COPD) (OR=1.76 for overall prognosis, OR=1.87 for mortality) were all associated with a poor prognosis. Obesity, however, did not have a significant impact. Diabetes also appeared to be strongly related to a poor prognosis, but the results were not statistically significant. The specific values of the ORs and 95% CIs can be found in Fig. 4. The findings for cardiovascular disease, hypertension, and diabetes were suspected to be influenced by publication bias, and the adjusted OR and 95% CI are provided in Supplementary Table 1 (in the online-only Data Supplement). Nonetheless, the results did not vary significantly.

Forest plot for comorbidities. Cardiovascular disease, hypertension, and chronic obstructive pulmonary disease were associated with a poor prognosis. Obesity had no significant effects. Diabetes seemed to be related to a poor prognosis, but it was not statistically significant [5,9-13,16,17,19,21,23]. CI, confidence interval.
Symptoms related to COVID-19
Fever and gastrointestinal symptoms did not significantly impact the prognosis, whereas respiratory symptoms, such as cough and dyspnea, were associated with patients’ prognosis. The prognosis was particularly poor when dyspnea was present (OR=5.20 for overall prognosis; OR=5.55 for mortality). Patients who needed oxygen supplementation also had a poor prognosis. The specific values of the ORs and 95% CIs are presented in Fig. 5.
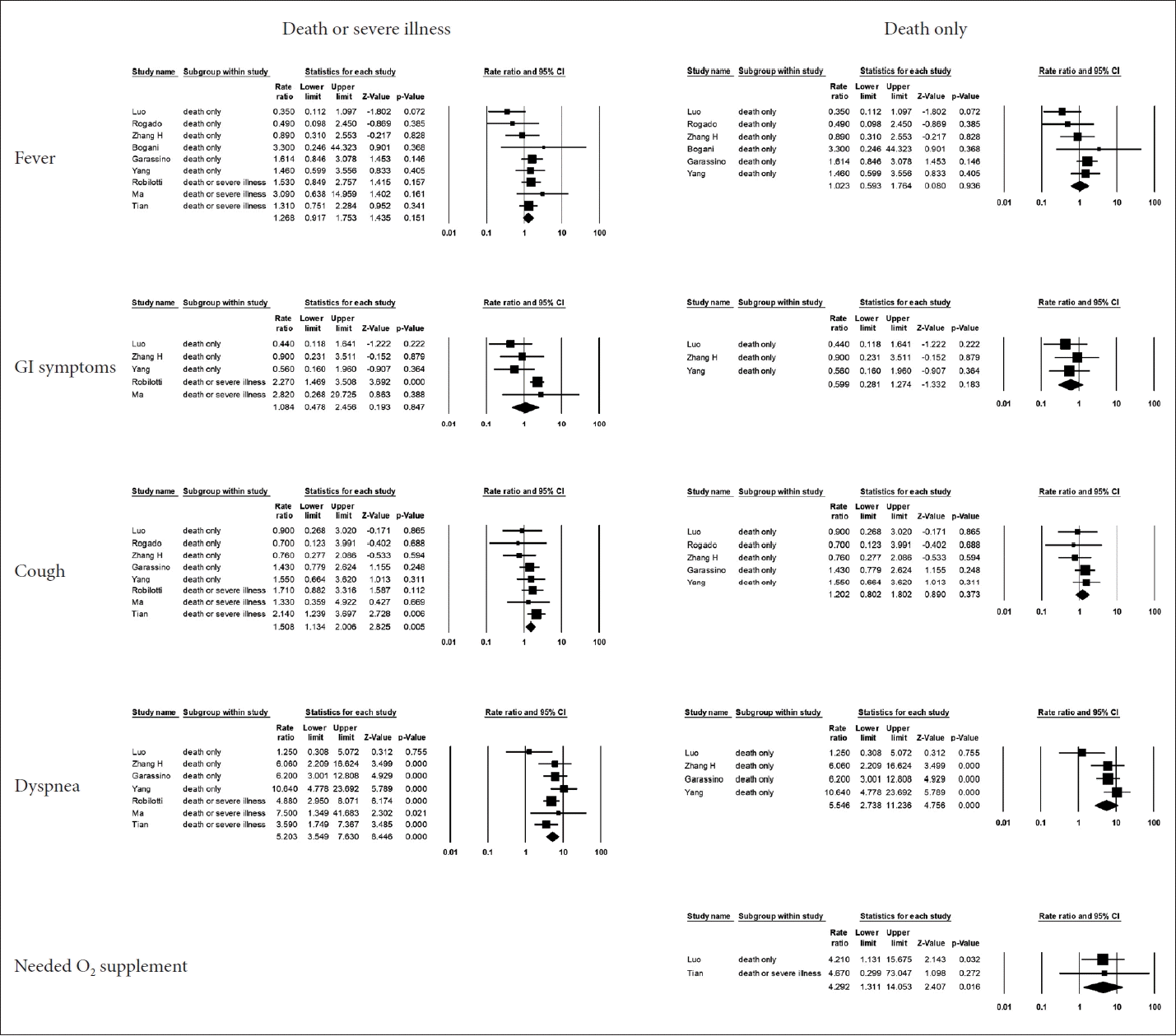
Forest plot for symptoms related to COVID-19. Fever and gastrointestinal (GI) symptoms did not affect the prognosis. Respiratory symptoms, including cough and dyspnea, were mainly associated with a poor prognosis. Patients who required oxygen (O2) supplementation also had a poor prognosis [12,13,15-17,19-21,23]. CI, confidence interval.
Cancer treatment within 4 weeks of COVID-19 diagnosis
No cancer treatments, including immunotherapy, targeted therapy, surgery, and radiotherapy, received within 4 weeks of the COVID-19 diagnosis were significantly related to patients’ prognosis. Although chemotherapy appeared to slightly worsen the prognosis, the difference was not statistically significant. The values of the ORs and 95% CIs are shown in Fig. 6A. The outcomes for immunotherapy, chemotherapy, and radiotherapy are suspected to have been influenced by publication bias, and the adjusted ORs and 95% CIs can be found in Supplementary Table 1 (in the online-only Data Supplement). Nonetheless, the results did not vary substantially.
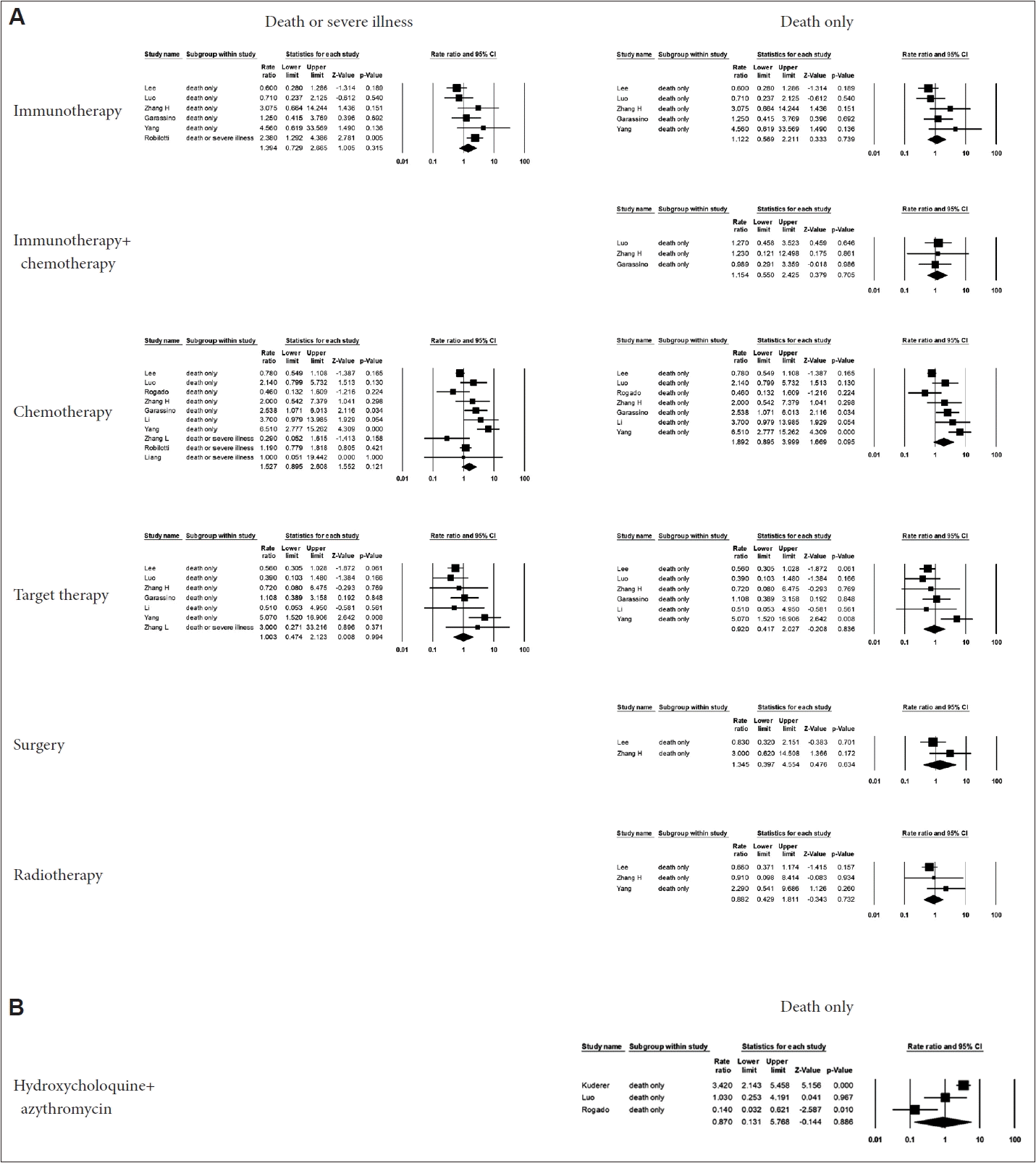
Forest plot for cancer treatment within 4 weeks of COVID-19 diagnosis. A: No cancer treatments including immunotherapy, targeted therapy, surgery, and radiotherapy received within 4 weeks of the COVID-19 diagnosis were related to patients’ prognosis. B: Forest plot for COVID-19 treatment. The combination of hydroxychloroquine and azithromycin had no significant effect on the prognosis [9-14,16,17,19,22,23]. CI, confidence interval.
Treatment for COVID-19 infection
The combination of hydroxychloroquine and azithromycin did not have a significant impact on prognosis (OR=0.87; 95% CI, 0.13–5.77), as shown in Fig. 6B.
DISCUSSION
COVID-19 is an acute respiratory disease caused by SARS-CoV-2. Cancer organizations worldwide have recently released care guidelines for cancer patients with COVID-19 [6-8]. This global trend highlights the significance of COVID-19 infection in individuals with cancer. Several studies have reported higher mortality rates in cancer patients with COVID-19 compared to non-cancer controls with COVID-19 [3-5]; however, drawing a solid conclusion is challenging due to most studies being single-center and conducted in one country, with the limitation of small sample sizes. Furthermore, various clinical prognostic factors have not been thoroughly investigated, although one systematic review compared laboratory findings between cancer patients and non-cancer controls infected with COVID-19 [24]. In this study, we gathered data from 3,215 cancer patients with COVID-19 across 16 studies from different countries and analyzed overall mortality following COVID-19 infection in cancer patients compared to controls, as well as prognostic factors for severity and mortality after COVID-19 infection [5,9-23]. The analyzed prognostic factors included demographics, comorbidities, COVID-19-related symptoms, cancer treatment within 4 weeks of COVID-19 diagnosis, and treatment for COVID-19 infection.
Piroth et al. [25] conducted a nationwide population-based cohort study comparing the mortality of COVID-19 and seasonal influenza, reporting that in-hospital mortality was higher in patients with COVID-19 (16.9%) than in patients with influenza (5.8%), with a relative risk of death of 2.9. Since most cancer patients experience systemic immunosuppression due to cancer itself or its treatment, it can be hypothesized that patients with cancer are more susceptible to COVID-19 infection and a fatal course than individuals without cancer. Higher mortality in cancer patients with COVID-19 has been reported in several studies. A retrospective case study in three different hospitals in China, which included 28 cancer patients with COVID-19, demonstrated a high mortality rate [9]. Miyashita et al. [4] found that in New York City, patients with cancer younger than 50 years had a significantly higher mortality rate, while the mortality rates of cancer patients with COVID-19 were lower in age groups older than 50 years. However, the results of this study were not statistically significant. A recent systematic review and meta-analysis by ElGohary et al. [24] examined the risk of COVID-19 infection in 1,108 cancer patients and reported a mortality risk of 21.1% (95% CI, 14.7%–27.6%), which aligns with our result (21% mortality). We can conclude that the mortality from COVID-19 (approximately 17%) is higher than that of patients with seasonal influenza (5.8%), and even higher in cancer patients (20%–21%) [24,25]. COVID-19 is associated with an inflammatory burst and lymphopenia, which may worsen cancer prognosis. However, it remains to be determined whether cancer alone is an independent risk factor for severe COVID-19. Emerging epidemiological studies have highlighted that delays in cancer diagnosis and treatment result in high mortality during the COVID-19 outbreak [26]. Therefore, physicians should be aware that the mortality of patients with cancer may have been significantly impacted not only by the viral disease itself but also by the extreme pressure exerted by COVID-19 on the healthcare system, leading to the postponement of cancer treatments and the allocation of scarce resources, such as intensive care beds and ventilators, to patients with better prognoses.
Male sex, older age, comorbidities like cardiovascular and respiratory diseases, smoking, and poor general performance status correlated with poor prognosis in patients with cancer [2]. Since cancer treatment-related, demographic factors, various symptoms, and other general patient factors seem to be important correlates of prognosis for patients with cancer who develop COVID-19 beyond cancer itself, we evaluated a number of prognostic factors affecting COVID-19 severity in cancer patients. First, we analyzed demographic factors and found that the prognosis of COVID-19 infection in cancer patients was poor in men and those aged ≥65 years. Compared to seasonal influenza, COVID-19 is likely to have a higher potential for respiratory pathogenicity, leading to more respiratory complications and higher mortality. Interestingly, patients admitted to the hospital with COVID-19 more frequently developed acute respiratory failure, pulmonary embolism, septic shock, or hemorrhagic stroke than patients with influenza [25]. Our analysis supported the finding that respiratory symptoms like cough and dyspnea, unlikely fever, or gastrointestinal symptoms significantly correlated with a poor prognosis. Moreover, even though only two studies evaluated the necessity of oxygen supplementation during the treatment process [13,21], patients who needed oxygen supplementation tended to have a poor prognosis, reflecting the close association between lung injury in COVID-19 and poor prognosis. Theoretically, cytokine burst-associated lung injury is a potential etiology in severe cases of COVID-19 [27]. Although COVID-19 can worsen underlying illnesses, only cardiovascular disease, hypertension, and COPD were related to poor prognosis in COVID-19 cancer patients in this meta-analysis.
Lou et al. [28] investigated the perspectives of cancer patients and their health during the COVID-19 pandemic, concluding that patients undergoing active cancer treatment were primarily concerned about the short-term effects of the pandemic on the logistics and potential efficacy of their ongoing treatment, its long-term effects, and broader societal concerns. The immunosuppression caused by some cancer treatments, such as chemotherapy, increases patients’ risk of contracting viruses and experiencing complications from COVID-19 compared to the general population [29-31]. Delays and alterations in cancer treatments due to the deprioritization of certain hospital activities considered non-urgent may also cause patients to worry about the impact of these changes on their cancer prognosis. The National Institutes of Health guidelines strongly recommend consulting with a hematologist or oncologist before adjusting cancer-related medication [32]. Zhang et al. [9] identified that recent use of anticancer therapies within 14 days of COVID-19 infection was an independent predictor of death or other severe events with a hazard ratio >4. Our results demonstrated that all cancer treatments, including immunotherapy, targeted therapy, surgery, and radiotherapy received within 4 weeks of COVID-19 diagnosis, were not significantly related to the patient’s prognosis. Although cancer patients are more likely to be infected than the general population and significantly more likely to die from COVID-19 once infected, the greater risk may be altering or foregoing cancer treatment. Intriguingly, antineoplastic therapies may either increase COVID-19 vulnerability or have a dual therapeutic effect on cancer and COVID-19 [33]. As the second wave of COVID-19 infections emerged, Bi et al. [34] concluded that recovered COVID-19 cancer patients remained negative for SARS-CoV-2 in the short term following chemotherapy.
According to the Centers for Disease Control and Prevention, the current clinical management of COVID-19 involves infection prevention, control measures, and supportive care, including supplemental oxygen and mechanical ventilatory support when necessary. The US Food and Drug Administration (FDA) has approved one drug, remdesivir (Veklury), for the treatment of COVID-19 in specific situations [35]. Initial trials for COVID-19 treatment involved chloroquine and hydroxychloroquine, with or without azithromycin, which have been studied in multiple clinical trials. Although early reports suggested that hydroxychloroquine might reduce the duration of SARS-CoV-2 infection and the severity of COVID-19 [36], subsequent studies failed to confirm the drug’s significant effects, indicating that the concurrent treatment of cancer and COVID-19 with hydroxychloroquine is not recommended [37,38]. Hydroxychloroquine, chloroquine, and azithromycin have not been approved by the FDA for the treatment of COVID-19. Despite hydroxychloroquine’s cardiac toxicity, such as QT prolongation, the combination of hydroxychloroquine and azithromycin did not significantly affect prognosis in this meta-analysis. In clinical settings, dexamethasone, a corticosteroid widely used in various medical applications, including cancer treatment, was recently found to reduce mortality in patients with COVID-19 who require respiratory support in a large randomized controlled trial, establishing its role in this context [39].
The present study had several limitations. First, the results of this meta-analysis were heterogeneous. Ideally, all studies used for meta-analysis should be conducted using the same protocol and in the same manner, but the study designs were different. To account for this heterogeneity as much as possible, we employed the DerSimonian-Laird random-effects model. Although detailed results were not presented in this study, the sensitivity test revealed that many items were significantly influenced by the results of individual papers, which was also due to the heterogeneity of the studies. Second, there was a possibility of publication bias. We detected publication bias using various statistical methods and discussed potential publication bias in Supplementary Fig. 1 (in the online-only Data Supplement). Additionally, we corrected the odds ratio by considering publication bias in individual analyses. Third, the specific cancer type and stage may be associated with risk factors for severe COVID-19 in the cancer patient population. Finally, some studies regarding COVID-19 infection in cancer patients are rapidly emerging and were not included in the present meta-analysis.
Based on our meta-analysis, we propose two major recommendations for cancer patients during the COVID-19 crisis. First, more intensive surveillance or treatment should be considered for cancer patients infected with COVID-19, particularly for older male patients, those with respiratory symptoms, or those with comorbidities such as cardiovascular disease, hypertension, and COPD. Second, timely cancer treatment should not be delayed during COVID-19 infection. Although several studies have investigated the impact of COVID-19 infection on cancer patients, caution is necessary when interpreting the findings, as the number of patients is small and data were collected retrospectively from a single country in some studies. To address these limitations, this is the first meta-analysis examining prognostic factors for severity and mortality following COVID-19 infection in cancer patients, drawing from 16 high-quality studies that encompass different races. Further research based on individual patient data is needed to better understand the risk of COVID-19 in cancer patients.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.18787/jr.2023.00015.
Notes
Ethics Statement
Not applicable
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Jae Hoon Cho who is on the editorial board of the Journal of Rhinology was not involved in the editorial evaluation or decision to publish this article. The remaining author has declared no conflicts of interest.
Author Contributions
Conceptualization: Eun Jung Lee, Jae Hoon Cho. Data curation: Eun Jung Lee, Jae Hoon Cho. Formal analysis: Jae Hoon Cho. Funding acquisition: Eun Jung Lee. Investigation: Eun Jung Lee, Jae Hoon Cho. Methodology: Jae Hoon Cho. Resources: Jae Hoon Cho. Supervision: Jae Hoon Cho. Validation: Eun Jung Lee, Jae Hoon Cho. Visualization: Eun Jung Lee, Jae Hoon Cho. Writing—original draft: Eun Jung Lee. Writing—review & editing: Jae Hoon Cho.
Funding Statement
This work was supported by a faculty research grant of Yonsei University Wonju College of Medicine (No. 2022-52-0054 granted to E.J.L.) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (2021R1A2C1010082, granted to E.J.L.).