INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleeprelated respiratory disorder, characterized by repeated episodes of hypopnea or apnea during sleep due to upper respiratory tract obstruction [
1]. Continuous positive airway pressure (CPAP) therapy is considered the gold-standard treatment for moderate to severe OSA [
2]. CPAP provides a constant amount of pressure greater than atmospheric pressure through a mask worn on the nose and mouth during inspiration and expiration to prevent upper airway collapse. Many effects of CPAP therapy have been described. The most important of these effects are a reduction in the apnea-hypopnea index (AHI), improved sleepiness, and improved overall quality of life [
3].
However, CPAP can cause uncomfortable or even lifethreatening side effects, although those side effects are rare. Trauma-related side effects can occur in CPAP treatment due to the high pressure to which patients are exposed. In rare cases, CPAP can cause pneumothorax, pneumomediastinum, and lung herniation associated with high pressure in the thorax [
4-
7]. These complications can occur in any patient, but in patients with lung disease, they are both more likely to occur and more life-threatening.
As reported herein, we recently experienced a rare complication of automatic positive airway pressure (APAP) therapy useŌĆönamely, pneumothorax in a 72-year-old male patient with OSA and chronic obstructive pulmonary disease (COPD). To the best of our knowledge, this is the first case report of pneumothorax after APAP use to be reported in a Korean journal.
CASE REPORT
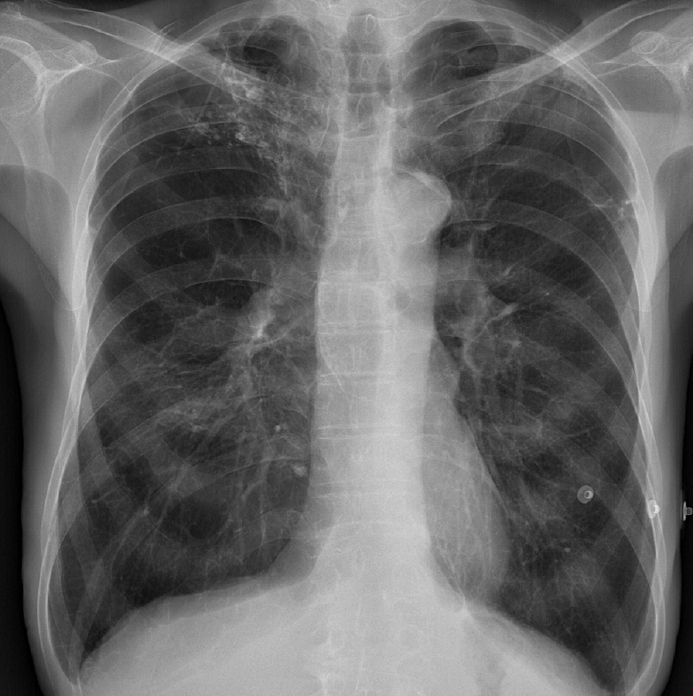
A 72-year-old male patient with a history of COPD and hypertension presented to our hospital for the evaluation and treatment of chronic snoring and mouth breathing. He had a history of pulmonary tuberculosis 40ŌĆō50 years ago. Four months before the patient visited the hospital, a chest X-ray was done, which showed fibrocalcific scarring in both upper lung fields, small calcified granulomas in the right lower lung field, and emphysematous changes in both lungs (
Fig. 1). The patient underwent polysomnography (PSG) for snoring. According to the results of PSG, the AHI was 41.2 per hour of total sleep time, and the lowest oxygen saturation was 83%. The patient had severe OSA and complained of prominent symptoms, such as snoring, so PAP therapy was recommended.
CPAP titration was done to identify the optimal pressure to prevent the occurrence of respiratory disorders. The AHI was 4 per hour under a pressure of 4 cm H2O, and the AHI was 0 under a pressure of 5 cm H2O. Therefore, the optimal CPAP titration pressure was determined to be 5 cm H2O.
The patient was started on APAP with a pressure range of 4ŌĆō8 cm H2O to treat OSA. After using APAP for 1 month, the patientŌĆÖs daytime sleepiness improved and the average AHI decreased to 4.7 per hour. After 2 months of APAP use, the average AHI decreased to 2.6 per hour. The 90th-percentile pressure was 5.3 cm H2O, and the air leak from the mask was <30 L/min.
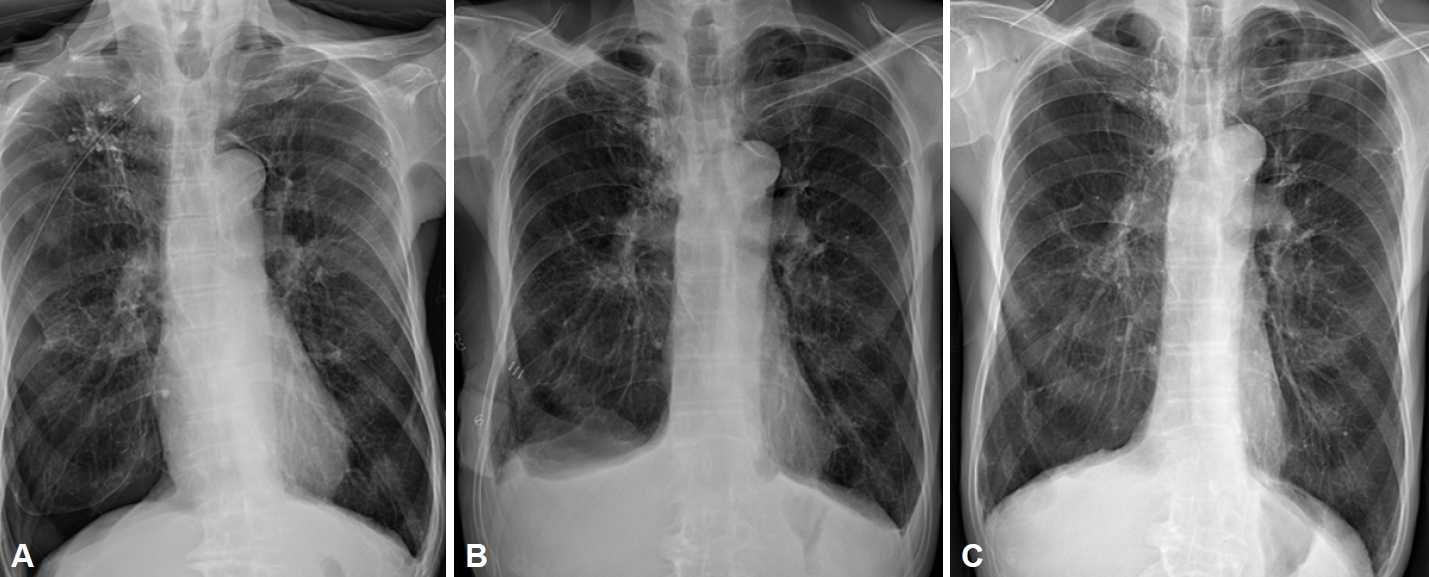
After 4 months of using APAP, the patient visited the emergency department with sudden severe dyspnea and chest discomfort at midnight. Dyspnea occurred 6 days before the patientŌĆÖs visit to our hospital, and he underwent right chest tube insertion and pleurodesis under pneumothorax diagnosis at another hospital. However, there was no improvement. Therefore, he was transferred to our hospital. His respiration rate increased to 24 breaths per minute, the oxygen saturation was 92% with decreased right lung sounds, and the chest tube was leaking. After the size of the chest tube was increased, the chest tube still leaked, and a chest X-ray showed pneumothorax (
Fig. 2A). One day after changing the chest tube, air leakage continued around the chest tube insertion site, and a chest X-ray showed that the amount of pneumothorax had increased. Therefore, video-assisted thoracic surgery (VATS) for right upper lobe and lower lobe wedge resection and talc pleurodesis were performed on the third day of hospitalization.
Two days after the operation, the patientŌĆÖs symptoms (dyspnea and chest discomfort) improved, and the air leak also improved. On a chest X-ray, although suspected pleural effusion was noted, the amount of pneumothorax decreased (
Fig. 2B). On the third day after surgery, lung expansion was maintained after chest tube clamping; therefore, the patient was discharged after chest tube removal.
Four months after surgery, the patient came into our hospital again for sleep apnea treatment. A chest X-ray showed that the pneumothorax had improved (
Fig. 2C). We requested a consultation with the pulmonology department, where a pulmonary function test was performed. The forced vital cavity (FVC) was 91%, forced expiratory volume in 1 second (FEV1) was 80%, and the FEV1/FVC ratio was 60%. Therefore, the pulmonology department indicated that although emphysema was severe on the chest X-ray, the decrease in lung capacity was not severe according to the pulmonary function test; therefore, the use of CPAP with a pressure of 5 cm H
2O would be a major problem. The patient thereafter used CPAP at a pressure of 5 cm H
2O. After 1 month of CPAP use, the patientŌĆÖs daytime sleepiness improved and the average AHI decreased to 2.9 per hour. We followed up with the patient for 1 year after surgery, and the patient maintained CPAP use without any complications.
DISCUSSION
Sleep apnea is a potentially life-threatening sleep disorder in which breathing stops and starts repeatedly during sleep [
1]. OSA is the most common type of sleep apnea. The potential benefits of successfully treating OSA include decreased cardiovascular morbidity and mortality [
8]. Positive airway pressure therapy is a major treatment for adults with OSA. CPAP provides continuous pressure during the entire breathing cycle, which maintains a positive pharyngeal transmural pressure, so that the intraluminal pressure exceeds the surrounding pressure. CPAP also stabilizes the upper airway by increasing the end-expiratory lung volume. This prevents respiratory events caused by upper airway collapse. CPAP is the most frequently used method because it is the simplest, the most familiar, and the best studied.
CPAP-related complications are rare. The side effects of CPAP treatment include interface-related side effects, pressure-related side effects, rhinorrhea, and congestion. Interface-related side effects include air leaking from the CPAP mask, skin marks, or rashes from CPAP masks. If a mask does not fit properly, air can leak around the edge of the mask, and marks can be left on the patientŌĆÖs skin, including sores and rashes. Pressure-related side effects include dryness of the mucosa, difficulty exhaling, an inability to sleep, musculoskeletal chest discomfort, aerophagia, and sinus discomfort.
Pneumothorax or pneumomediastinum also can rarely occur after the use of PAP. Previously reported cases of pneumothorax after the use of noninvasive positive pressure ventilation for OSA are summarized in
Table 1.
Rajdev et al. [
7] reported a case of frequent pneumothorax due to APAP treatment. The patient was started on APAP with a pressure range of 4ŌĆō20 cm H
2O. Although pneumothorax occurred after 2 months of treatment, APAP treatment was continued after the end of pneumothorax treatment. Recurrence was noted 6 months after the occurrence of pneumothorax; therefore, chest tube insertion was done and APAP was discontinued. After 2 weeks, the pneumothorax improved. APAP was therefore resumed with the same pressure settings. The 95th-percentile pressure was 14.8 cm H
2O before the first incidence of pneumothorax and 16.8 cm H
2O before the second occurrence of pneumothorax. Pneumothorax recurred 12 days after APAP treatment was resumed. So chest tube insertion was performed, and APAP treatment was discontinued. When positive airway therapy was resumed after the first treatment of pneumothorax, it is thought that changing the treatment to CPAP or bilateral positive airway pressure rather than APAP could prevent excessive positive pressure and subsequent pneumothorax recurrence.
Pneumothorax in lungs previously damaged by emphysema is more severe than in otherwise healthy lungs. Most clinically stable patients with secondary spontaneous pneumothorax should be treated with a catheter or chest tube thoracostomy. In another study, the symptoms improved after chest tube insertion [
9]. However, if the air leak is prolonged, the chest tube can be changed to a larger one, or VATS surgery or pleurodesis can be performed. The patient presented herein is the only one who underwent surgery for pneumothorax.
Pulmonary barotrauma can occur as a result of a continuous increase in pressure, which causes hyperinflation and rupture of alveoli in CPAP-treated patients [
4]. In particular, patients with emphysematous lungs have a higher likelihood of developing pneumothorax because their function is already impaired. Therefore, patients receiving CPAP treatment should be closely monitored for both their underlying diseases and CPAP complications. If patients have respiratory diseases, a chest X-ray examination and lung function tests should be performed before CPAP treatment. Even when there is no reported underlying lung disease, an evaluation for respiratory function is recommended, especially in Korea, because the prevalence of COPD is 21.6% and 5.8% in Korean men and women, respectively [
8]. After testing, a consultation with pulmonology should be done to discuss whether CPAP is suitable and, if so, the appropriate CPAP pressure. After starting CPAP, we should continue to monitor patients for specific symptoms, such as difficulty breathing or chest pain, and if such symptoms occur, we can suspect pneumothorax.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print