Comparison of Outcomes After Septoplasty With Non-Absorbable or Biodegradable Synthetic Polyurethane Foam Nasal Packing With a Focus on Pain and Cardiac Factors
Article information
Abstract
Background and Objectives
We compared pain levels, cardiovascular parameters, and complications according to whether patients underwent nasal packing with non-absorbable or biodegradable materials.
Methods
Patients who underwent septoplasty from May 2015 to April 2020 were retrospectively reviewed. Numeric rating scale (NRS) scores for pain, blood pressure, and heart rate were measured three times (immediately after surgery, 6 hours later, and on postoperative day [POD] 1). We collected data on complications, including postoperative bleeding, septal hematoma, adhesions, septal perforation, and the recurrence of septal deviation.
Results
In total, 200 patients underwent septoplasty, of whom 100 underwent nasal packing with Merocel and 100 underwent packing with Nasopore. The summed NRS scores over the three time points did not differ significantly between the groups. The NRS scores at 6 hours after surgery were highest in both groups. The systolic and diastolic blood pressure and the heart rate immediately after surgery were significantly higher than before surgery in both groups. The blood pressure and heart rate at 6 hours after surgery and on POD 1 did not differ significantly from those before surgery in either group. The incidence of sleep disturbance, postoperative bleeding, septal hematoma, adhesions, septal perforation, and recurrence of septal deviation did not differ significantly between the two groups.
Conclusion
Although the level of postoperative pain and the cardiovascular parameters changed over time, we found no significant differences in pain, blood pressure, heart rate, or the complication rate according to whether patients underwent nasal packing with Nasopore or Merocel.
INTRODUCTION
Septoplasty is one of the most common operations performed by otorhinolaryngologists, and septal hematoma, postoperative bleeding, and mucoperichondrial flap instability are concerns. Trans-septal suturing is commonly employed to minimize those complications [1]. Several reports have compared different types of nasal packing after septoplasty and evaluated the effects of trans-septal suturing. The meta-analysis of Kim and Kwon [2] found no significant among-group differences in terms of postoperative bleeding or the incidence of septal hematoma. However, Dadgarnia et al. [3]reported more severe postoperative bleeding in a transseptal suturing group. Nevertheless, some surgeons employ both nasal packing and trans-septal suturing with the goal of reducing postoperative complications.
Nasal packing induces nasal obstruction and mucosal irritation; patients experience nasal congestion, pain, and sleep disturbance immediately after surgery. Most reports found that patients who underwent nasal packing complained of more pain and headache than did those who underwent trans-septal suturing alone [4-7]. The removal of packing material is extremely painful. Pain and nasal resistance are expected to increase the blood pressure and heart rate.
Yilmaz et al. [8] reported that patients who underwent septoplasty followed by biodegradable nasal packing experienced less pain and bleeding than did those who underwent packing with non-absorbable material. We expected that the extent of pain and cardiovascular change would vary according to the packing material used.
The purpose of this study was to compare the pain levels, cardiovascular parameters, and complications according to whether patients underwent nasal packing with non-absorbable or biodegradable material.
METHODS
This study and the associated chart review were approved by the Institutional Review Board of the Catholic University of Korea, Seoul St. Mary’s Hospital, College of Medicine (approval no. KC20RISI0345). The requirement for written informed consent was waived by the IRB. Patients who underwent septoplasty with non-absorbable or biodegradable synthetic polyurethane foam (SPF) nasal packing at our hospital from May 2015 to April 2020 were retrospectively reviewed. Patients who underwent revision surgery or endoscopic sinus surgery, had a history of trauma, or had another sinus disease (e.g., benign or malignant neoplasm) or hematologic disorder were excluded. We collected demographic data and noted all complications, incidences of sleep disturbance, pain scores based on a numeric rating scale (NRS), blood pressure, and the heart rate.
All operations were performed by two surgeons assisted by residents. Intravenous flomoxef was administered at 30 minutes preoperatively. After general anesthesia was induced, 1% lidocaine with epinephrine was injected into the septal mucosa to facilitate hydrodissection. Standard septoplasty was performed endonasally, and the mucoperichondrial and mucoperiosteal flaps remained intact. After removing the deviated portion, septal quilting with 4-0 Vicryl or 5-0 polydioxanone was used to approximate the subperichondrial flaps. Before the operation ended, nasal packing was placed between the septum and inferior turbinate. Patients who received a non-absorbable material (Merocel; Medtronic Xomed Surgical Products, Jacksonville, FL, USA) constituted Group A, and those who received a biodegradable material (Nasopore; Polyganics, Groningen, the Netherlands) comprised Group B. Ten-centimeter-long sticks of Merocel were inserted individually into each nose. One-third of 8-cm-long Nasopore was cut, and one-third of these sticks was inserted into the concave side of the nasal cavity and two-thirds into the convex side. Merocel packing was removed on the morning of postoperative day (POD) 1. Patients with Nasopore packing were discharged on POD 1 without removing the packing.
NRS scores for pain and data on blood pressure and the heart rate were obtained immediately after surgery, 6 hours later, and before packing removal at 6 AM on the next day. Sleep disturbance was considered present if a patient woke up with pain and took a painkiller. We also collected data on complications including postoperative bleeding, septal hematoma status, adhesions, septal perforation status, and the recurrence of septal deviation.
Numerical variables are expressed as the means±standard deviations. The Student t-test was used to compare the NRS scores, blood pressure, and heart rate between the two groups; the paired t-test was employed to compare preoperative and postoperative data. The chi-square and Fisher exact tests were used to compare categorical variables (sex, sleep disturbance, postoperative bleeding, septal hematoma status, adhesion, septal perforation status, and the recurrence of septal deviation). A p-value <0.05 was considered to indicate statistical significance. All statistical analyses were performed with the aid of SPSS software version 24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
A total of 200 patients (32 [16%] males and 168 [84%] females) who underwent septoplasty from May 2015 to April 2020 were included. The mean patient age was 33.5±15.4 years (range, 12–78 years). The 100 patients (21 males, 79 females) who received Merocel packing constituted Group A and the 100 patients (11 males, 89 females) who received Nasopore packing comprised Group B. The mean patient age was 35.2± 15.9 years in Group A and 31.7±14.6 years in Group B. There was no significant difference between the two groups in age, sex ratio, or history of hypertension.
The NRS pain score in Group A was 3.4±2.4 immediately after surgery, 3.8±2.3 6 hours later, and 2.4±2.0 the next morning. The corresponding Group B scores were 2.4±1.8, 4.5±2.3, and 3.0±1.8, respectively (Fig. 1A). The score was significantly higher in Group A than in Group B immediately after surgery (p=0.001). However, Group B had slightly higher scores at 6 hours after surgery (p=0.037) and on POD 1 (p=0.039). The summed NRS scores over the three time points did not differ significantly between the two groups (9.6±5.0 vs. 9.9±3.8, p=0.717). The NRS scores at 6 hours after surgery were the highest of all three scores in both groups.
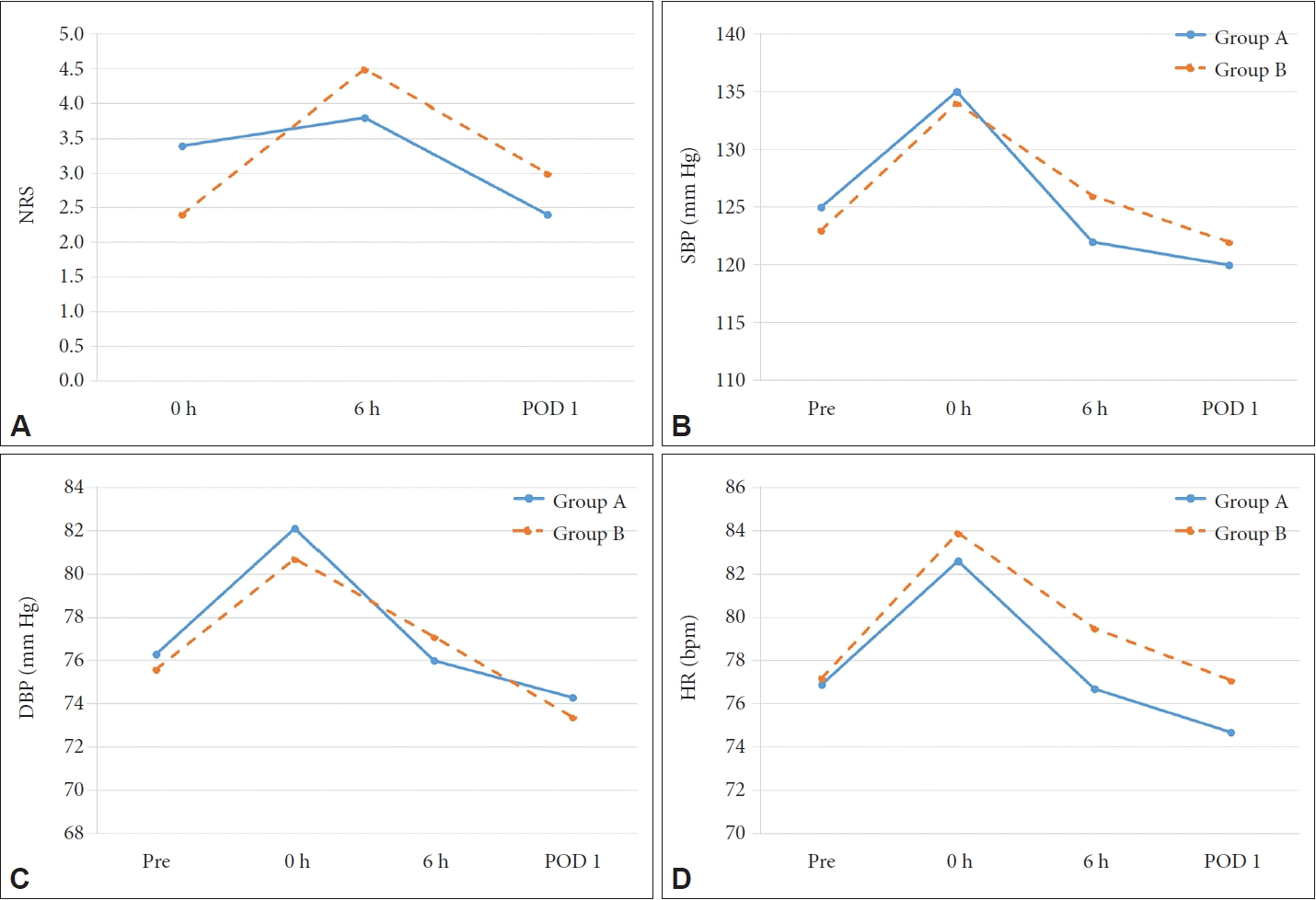
Pain levels and cardiovascular parameters in the two groups. A: NRS pain scores. B: Systolic blood pressure. C: Diastolic blood pressure. D: Heart rate. NRS, numeric rating scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
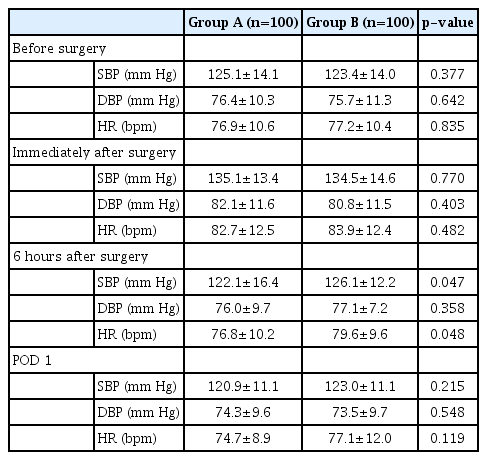
The mean systolic blood pressure before surgery was 125.1± 14.1 mm Hg in Group A and 123.4±14.0 mm Hg in Group B. The mean diastolic blood pressure before surgery was 76.4±10.3 mm Hg in Group A and 75.7±11.3 mm Hg in Group B. The mean heart rate before surgery was 76.9±10.6 beats per minute (bpm) in Group A and 77.2±10.4 bpm in Group B. The mean systolic blood pressure immediately after surgery was 135.1±13.4 mm Hg in Group A and 134.5±14.6 mm Hg in Group B. The systolic blood pressure immediately after surgery was significantly higher in both groups than before surgery (p<0.001). The diastolic blood pressure and heart rate immediately after surgery were also significantly higher than before surgery in both groups (p<0.001). However, the blood pressure and heart rates at 6 hours after surgery and on POD 1 did not differ significantly from those before surgery in either group (Fig. 1B-D). Significant between-group differences in systolic and diastolic blood pressure and heart rate were only evident at 6 hours after surgery (Table 1).
Sleep disturbance caused by pain, as well as complications such as postoperative bleeding, septal hematoma, adhesions, septal perforation, and the recurrence of septal deviation, did not differ significantly between the two groups (Table 2).
DISCUSSION
Nasal packing was first described in 1951 [9]; since then, clinicians have sought to minimize pain, nasal obstruction, and sleep disturbance using various materials or (alternatively) techniques other than packing. Merocel often serves as the packing material after septoplasty, since it is cheap, shows high elasticity when wet, and provides stable support to the nasal cavity [10,11]. However, Merocel must later be removed, which is very painful [12]. Thus, biodegradable packing materials such as SPF have been used to reduce pain and improve patient comfort and mucosal healing. SPF drains from the nasal cavity in mucus or upon saline irrigation. Yilmaz et al. [8] reported that an SPF-packing group reported significantly less pain than a Merocel-packing group even during packing. Kim et al. [13] found that an SPF group experienced significantly less packing pain (because of the absence of removal), but the pain during packing was similar in those patients and in patients who underwent packing with a non-resorbable material. We evaluated pain at three time points. Immediately after surgery, Group A reported more pain than Group B, which was attributable to Merocel-induced nasal obstruction and pressure. In contrast, Nasopore was used to pack only one-third or two-thirds of each nasal cavity. Airflow was thus possible immediately after surgery. However, at 6 hours after surgery and on POD 1, the pain in Group B was similar to that in Group A because the injury to and stimulation of the nasal mucosa during surgery increased venous engorgement, nasal secretions, mucosal swelling, edema, and nasal obstruction [14]. Thus, the summed NRS scores over the three time points did not differ significantly between the two groups (p=0.717).
Pain in both groups increased immediately after surgery, peaked 6 hours later, and then decreased, as observed on POD 1. Nasal congestion and pressure developing after surgery increased the pain level. Patients with high-level anxiety reported more postoperative pain [15,16]. Ploghaus et al. [17] found that anxiety increased pain severity and perceived discomfort, and hippocampal activity reduced the pain threshold by facilitating activation of the entorhinal cortex. Kayabasi et al. [18] found that postoperative 6 hours visual analog scale pain scores were significantly (p<0.001), positively, and strongly correlated with Hospital Depression and Anxiety Scale scores obtained at 1 hour before septoplasty. Thus, several factors combine to increase the pain level. Postoperative pain affects patients’ functional satisfaction. Gadkaree et al. [19] analyzed the relationship between postoperative pain and patient satisfaction with the functional outcomes after functional rhinoplasty. Patients who experienced less pain reported better functional improvements (e.g., in breathing) than expected (p=0.001). Therefore, patient satisfaction can be improved by controlling pain at 6 hours after surgery.
The nasal obstruction caused by nasal packing induces mouth-breathing, which is not physiological and requires more energy than nasal breathing [20]. The level of oxygen entering the lungs decreases during mouth-breathing because the respiratory muscles do not adequately support such breathing. Rapid breathing does not allow the lung dead space to fill with air and compromises gas exchange in the alveoli [21]. The resultant hypoxia increases the blood pressure and heart rate. Many reports have been published on blood gas changes caused by nasal packing. Cook and Komorn [22] reported a significant decrease in the PO2 and an increase in the PCO2 after nasal packing. Yildirim et al. [23] compared groups that underwent nasal packing and septal suturing; significant hypoxia and hypercapnia were evident in only the former group. We found significant increases in the systolic and diastolic blood pressure and heart rate immediately after surgery in both groups (all p<0.001). Gal and Cooperman [24] reported that extubation after general anesthesia promoted postoperative hypertension, pain, hypercapnia, bladder distension, hypothermia, shivering, and tracheal irritation. We consider that the observed elevations in blood pressure and heart rate were attributable to both hypoxia and the other factors listed above.
The blood pressure and heart rate were expected to remain high because of the pain associated with nasal packing; however, all values gradually decreased (in both groups) at 6 hours after surgery and on POD 1, reaching levels similar to or below those before surgery. Taheri et al. [25] compared the blood pressure and heart rate before and at 24 hours after surgery between patients who underwent nasal packing and those who did not. No significant differences were found either before or after surgery. Zayyan et al. [26] used a 24-hour Holter device to compare the heart rate before and after surgery involving nasal packing. The mean heart rate did not differ significantly between an airflow-packing group and a glove-finger-packing group. The decreases in blood pressure and heart rate are attributable to the nasocardiac reflex, irrespective of the pain and hypoxia levels. The nasocardiac reflex was discussed by Baxandall and Thorn [27]; profound bradycardia developed when a nasal speculum was inserted into the naris and turbinate and then manipulated with the patients under general anesthesia. Betlejewski et al. [28] triggered the reflex by stimulating the medial turbinate mucosae of 80 patients with 25% (v/v) ammonia; this significantly decreased the heart rate. The nasal cavity receives many sensory inputs via the ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve. The afferent limb reflex arc involves the V1 or V2 branches of the trigeminal nerve. This arc runs through the pterygopalatine and Gasserian ganglia, trigeminal nerve, sensory nucleus of that nerve, short internuncial fibers, motor nucleus of the vagus nerve, and (finally) the vagus nerve, thus affecting the heart [29]. Therefore, stimulation of the trigeminal nerve branch in the nasal cavity triggers parasympathetic responses such as bradycardia and hypotension. We found that both Merocel and Nasopore irritated the nasal mucosa, hence reducing the blood pressure and heart rate.
SPF packing did not lead to more comfortable sleep compared to Merocel. Kim et al. [13] and Yilmaz et al. [8] found no significant difference in terms of sleep disturbance between SPF and Merocel. SPF causes nasal fullness and pain, as does Merocel. We found no significant difference in sleep disturbance between the two groups.
The levels of postoperative complications (bleeding, septal hematoma, adhesions, septal perforation, and recurrence of septal deviation) did not differ significantly between the two groups. The complication rates were <6%, and those of septal hematoma and bleeding were <2%. SPF controlled bleeding and hematoma reliably, as did Merocel. The prevention of septal hematoma and postoperative bleeding is aided not only by nasal packing but also by trans-septal suturing. Three meta-analyses have compared nasal packing and trans-septal suturing after septoplasty. No significant difference in either the septal hematoma or postoperative bleeding rate was evident between patients who underwent nasal packing and those who underwent trans-septal suturing [2,7,30]. In our study, Nasopore was inserted partially in the nasal cavity, so it was not strong enough to press the septum, unlike Merocel. We consider that both groups exhibited similar outcomes because all patients underwent trans-septal suturing.
Our work had several limitations. First, the results of retrospective studies are not as definitive as those of randomized controlled studies. Second, septoplasty was performed by two surgeons. Although the procedures were similar, certain variables (such as the suture material used) were less controlled than would have been the case if only one surgeon had performed all procedures. Third, pain, blood pressure, and the heart rate were not measured after the removal of the packing material. However, we compared patients’ symptoms, changes in cardiovascular parameters, and complications when different packing materials were used. This might help surgeons choose an appropriate packing material after septoplasty.
In conclusion, we found that postoperative pain and cardiovascular parameters changed over time, but pain, blood pressure, the heart rate, and the complication rate did not differ significantly according to whether Nasopore or Merocel was used. We consider that the overall surgical satisfaction of patients will improve with precise pain control and the selection of appropriate (patient-specific) packing material.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Do Hyun Kim and Il Hwan Lee who are on the editorial board of the Journal of Rhinology were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Il Hwan Lee, Sung Won Kim. Data curation: Jae Seong An, Do Hyun Kim. Formal analysis: Do Hyun Kim. Investigation: Jae Seong An, Do Hyun Kim. Methodology: Soo Whan Kim. Software: Do Hyun Kim. Supervision: Il Hwan Lee, Sung Won Kim. Validation: Jae Seong An, Do Hyun Kim, Soo Whan Kim. Visualization: Do Hyun Kim. Writing—original draft: Jae Seong An. Writing—review & editing: Jae Seong An, Il Hwan Lee, Sung Won Kim.
Funding Statement
None