|
 |
| J Rhinol > Volume 29(2); 2022 |
|
Abstract
Rhino-orbito-cerebral mucormycosis (ROCM) is an invasive fungal infection that usually occurs in immunocompromised patients. It is aggressive and has a high risk of mortality. With unclear guidelines, ROCM is treated in various ways. We present a patient who underwent kidney transplant and who treated for ROCM without major complications.
Invasive fungal disease is an important cause of death in immunocompromised patients. Mucormycosis is an infection caused by a member of the order Mucorales found in the residues of plants, soil, and decaying vegetation. Most cases of mucormycosis result from inhalation of fungal sporangiospores that have been released into the air or direct inoculation of organisms into disrupted mucosa of the oral and nasal cavities [1].
Predisposing immunocompromising conditions, such as poorly controlled diabetes mellitus, hematologic malignancy, severe trauma, burn, and organ transplantation, are the main risk factors for mucormycosis [2]. Mucormycosis can have clinical manifestations in various organs, including the nasal cavity, pulmonary systems, central nervous system, and gastrointestinal system. The most common clinical presentation of mucormycosis is a rhino-orbito-cerebral infection [3]. The symptoms of rhino-orbito-cerebral mucormycosis (ROCM) include purulent discharge, perinasal cellulitis, facial pain, headache, lethargy, visual loss, proptosis, and cranial nerve involvement in severe cases.
The potential for permanent disability after aggressive surgical management presents a significant challenge for both doctors and patients. In this study, we present the case of a ROCM in a patient who had undergone kidney transplantation.
A 53-year-old woman visited the hospital with left orbital swelling and bloody rhinorrhea that had lasted for a month (Fig. 1A). She had a history of high blood pressure and, hyperthyroidism and had received an ABO-incompatible kidney transplant from her husband 2 years earlier for chronic renal failure. A month after receiving the kidney transplant, she had an acute transplant rejection. Since then, she has been on dialysis three times a week, with continuous administration of prednisolone (Solondo 5 mg tab qd) and tacrolimus (Tacrobell cap 1.5 mg bid). In addition, she was hospitalized for antibiotics once a year for recurrent pneumonia and urinary tract infection after kidney transplant. She had no fever, but her absolute neutrophil count was 264/μL.
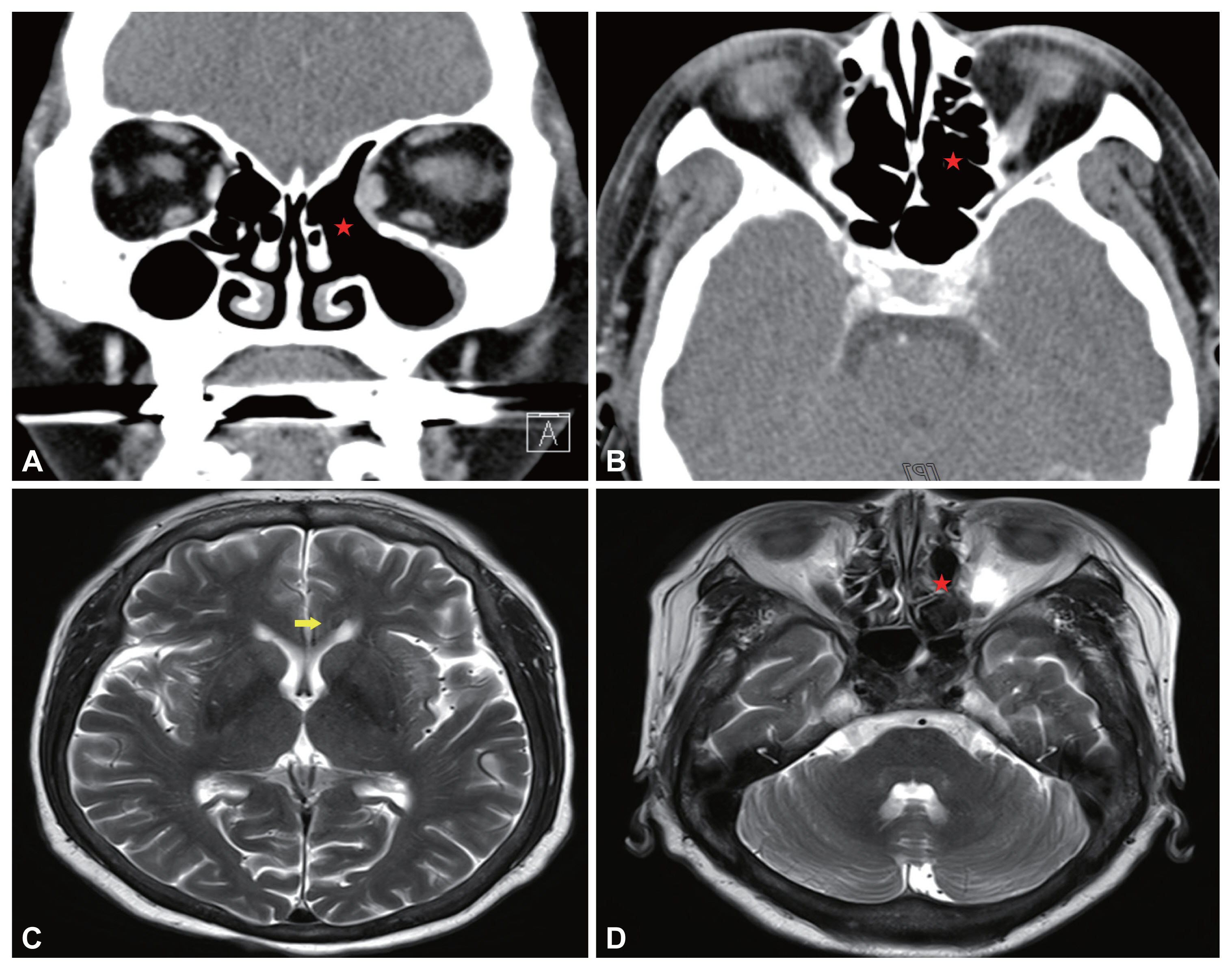
Ophthalmologic examination revealed cortical opacity and chemosis in the right eye. The extraocular movement was normal and there was no significant change in the visual acuity 0.5 of the right eye; the visual acuity of the left eye was 0.4 on the left side. A bulging mass filling the left middle meatus was observed during nasal endoscopy (Fig. 1B). There were no abnormal findings on neurological examination. Computed tomography (CT) revealed an unevenly enhanced soft tissue with calcification in the left ethmoid sinus. The 3.4-cm mass, which destroyed the cribriform plate and lamina papyracea, invaded the left medial rectus muscle and blocked the maxillary ostium (Fig. 2A and B). T2-weighted brain magnetic resonance imaging (MRI) showed a 34-mm heterogeneous enhancing lesion with clear boundaries involving the left ethmoid sinus and extending into the left orbit and an enhancing discrete lesion with an 8-mm rim in the left aspect of the genu of the corpus callosum (Fig. 2C and D).
Endoscopic sinus biopsy was performed, and irregular, ribbon-like hyphae, compatible with mucormycosis, were revealed by pathological examination (Fig. 3). When we considered the extent and mortality rate of ROCM, the patient was a candidate for enucleation of the left eye and craniotomy. However, because of her poor general condition, we decided to perform endoscopic sinus surgery using a navigation system (Medigator®). When uncinectomy was performed, purulent secretions with dark brown mud-like lesions were observed within the ethmoid sinus (Fig. 4A). Necrotic granulation tissue with thick purulent discharge was attached to the cribriform plate and lamina papyracea. When all necrotic tissue around the cribriform plate was removed, cerebrospinal fluid was not observed. After removing the necrotic tissue around the eyeball, we confirmed that the lamina papyracea was damaged and the periorbita was exposed. When the periorbita was sharply incised, a large amount of pus was found inside the eyeball with fat exposure. After sufficiently washing the inside of the eye ball, the surgery was completed after packing in the nasal cavity. The orbital swelling improved postoperatively, and there were no abnormalities of the extraocular movements and visual acuity.
Liposomal amphotericin B was administered as an intravenous infusion at 25 mg for 3 months postoperatively. Six months after surgery, the patient did not complain of any discomfort, and no abnormal findings were observed during the endoscopic evaluation (Fig. 4B). No lesions were observed on CT either (Fig. 5A and B). A decrease in the size of the lesion within the left aspect of the genu of the corpus callosum was revealed by follow-up MRI (Fig. 5C and D).
Despite treatment, early detection and aggressive management are essential for ROCM due to its high mortality. Traditional treatment options encourage complete removal of the lesions and debridement of necrotic tissue until viable bleeding tissue is encountered, often leading to orbital exenteration and skull base resection, in addition to systemic antifungal therapy [4].
Invasive fungal sinusitis is a rare condition that usually occurs in immunocompromised patients and often presents as a ROCM. The fungus invades the wall of the blood vessels, causing mechanical and toxic damage to the intima—and leading to thrombosis—associated tissue infarction and necrosis [5]. The infection progressively spreads from the nasal mucosa to the sinuses, palate, orbits and brain. It evolves in three stages: nasal and sinus involvements that are often asymptomatic and unnoticed by the patient, orbital involvement motivating the patient for consultation, and finally cerebral involvement. Early diagnosis, aggressive debridement of necrotized tissues, and antifungal therapy are important factors for successful treatment of ROCM, which can reduce the associated morbidity and mortality [2].
The management of mucormycosis involves the initiation of systemic antifungal agents. Amphotericin B has the broadest spectrum of activity against all available systemic antifungal agents. Its binding affinity for ergosterol is considered the primary reason for its antifungal efficacy, and its cholesterol-binding activity may be the source of toxicity [6]. Nephrotoxicity, which often requires the discontinuation of therapy, has been reported in almost 90% of patients who receive amphotericin B. Liposomal amphotericin B is often the antifungal agent of choice due to its superior survival rates and the ability to achieve higher blood levels with fewer nephrotoxic side effects [7]. Our patient had received a kidney transplant but was on dialysis due to acute transplant rejection. Therefore, she was treated with liposomal amphotericin B, and no major complications occurred.
Exenteration may be recommended for progressive orbital disease; however, there are no clear guidelines about its optimal timing. Although potentially life-saving, exenteration causes blindness, cosmetic disfigurement, and psychological trauma. In this paper, we report the successful management of an immunocompromised ROCM patient without exenteration. DiBartolo et al. [8] injected intravenous antifungal agents and performed a series of surgeries that ultimately resulted in orbital exenteration to preserve the life of ROCM patients with acute visual loss, unilateral facial edema, fixed dilated right pupil, and loss of extraocular movements. Even if the lesion invaded the eyeball, there was no restriction on extra ocular movement or impaired visual acuity in our patient, which enabled good treatment results. Blitzer et al. [9] suggested that the decision to proceed with exenteration for orbital involvement, even with cranial nerve involvement, depends on the aggressiveness of presentation, the underlying disease process, and the response to initial therapy. As such, we recommend the treatment of underlying diseases and somewhat preservative initial therapy for ROCM patients.
Clinically, ROCM can be characterized by rhinosinusitis with purulent discharge, nasal ulceration, epistaxis, facial pain with swelling, headache, ophthalmoplegia with blindness, proptosis, and decreased mental function. In a retrospective review of 23 patients, DelGaudio et al. [10] concluded that a radiological diagnosis of invasive fungal sinusitis should be considered in unilateral cases with more than expected mucosal edema. Osseous erosion and extra-sinus extension are late radiological signs. For the patient’s prognosis, we always need to evaluate the patient’s history and, the presence or absence of ROCM suspicious symptoms, and carefully perform physical and radiological examination.
Notes
Ethics Statement
The study was approved by the Gachon University Gil Hospital Clinical Research Ethics Review Committee (GDIRB2021-027). The written informed consent was obtained from a participant.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Fig. 1
Preoperative patient’s face pictures. Left periorbital swelling was observed. A: Patient’s left nasal cavity. B: A bulging mass filling the left middle meatus.

Fig. 2
Pre-operative patient’s paranasal sinus computed tomography view. A: Coronal view. B: Axial view. The 3.4-cm heterogenous mass was located inside of left ethmoid sinus. The lesion was accompanied by left orbital medial wall erosion and involved the medial rectus mscule (indicated by stars). C, D: Pre-operative patient’s paranasal sinus magnetic resonance imaging T2-weighted view. C: The 8-mm rim enhanced lesion was observed in left. aspect of genu of the corpus callosum (indicated by arrow). D: The heterogeneous enhancing lesion inside the left ethmoid sinus has a clear boundary with the inside of the nasal cavity, but confirmed as a pattern invading the left orbital wall (indicated by star).
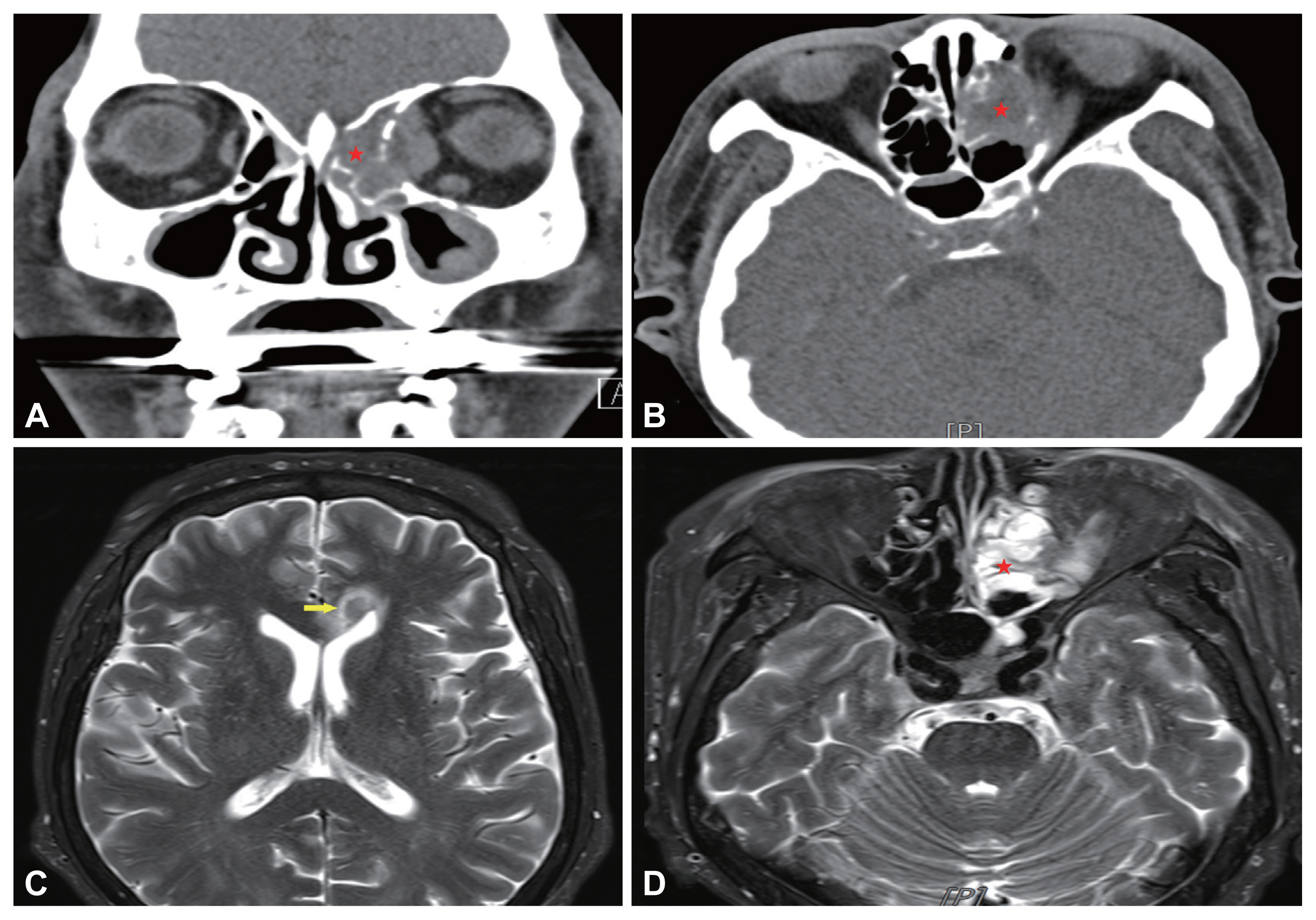
Fig. 3
Pathology of left. Nasal cavity lesion biopsy. A number of irregular shaped of ribbon-like hyphae are observed in H&E stain ×200 (A), GMS stain ×400 (B). H&E, Hematoxyling and Eosin; GMS, Grocott’s Methenamine Silver.

Fig. 4
Intra-operative. The dark brown mud-like discharge was shown from the inside of the ethmoid sinus. A: Necrotic granulation tissue with thick purulent discharge was attached to the cribriform plate and lamina papyracea. B: Three months after surgery, there were no abnormal findings on patient’s nasal cavity.
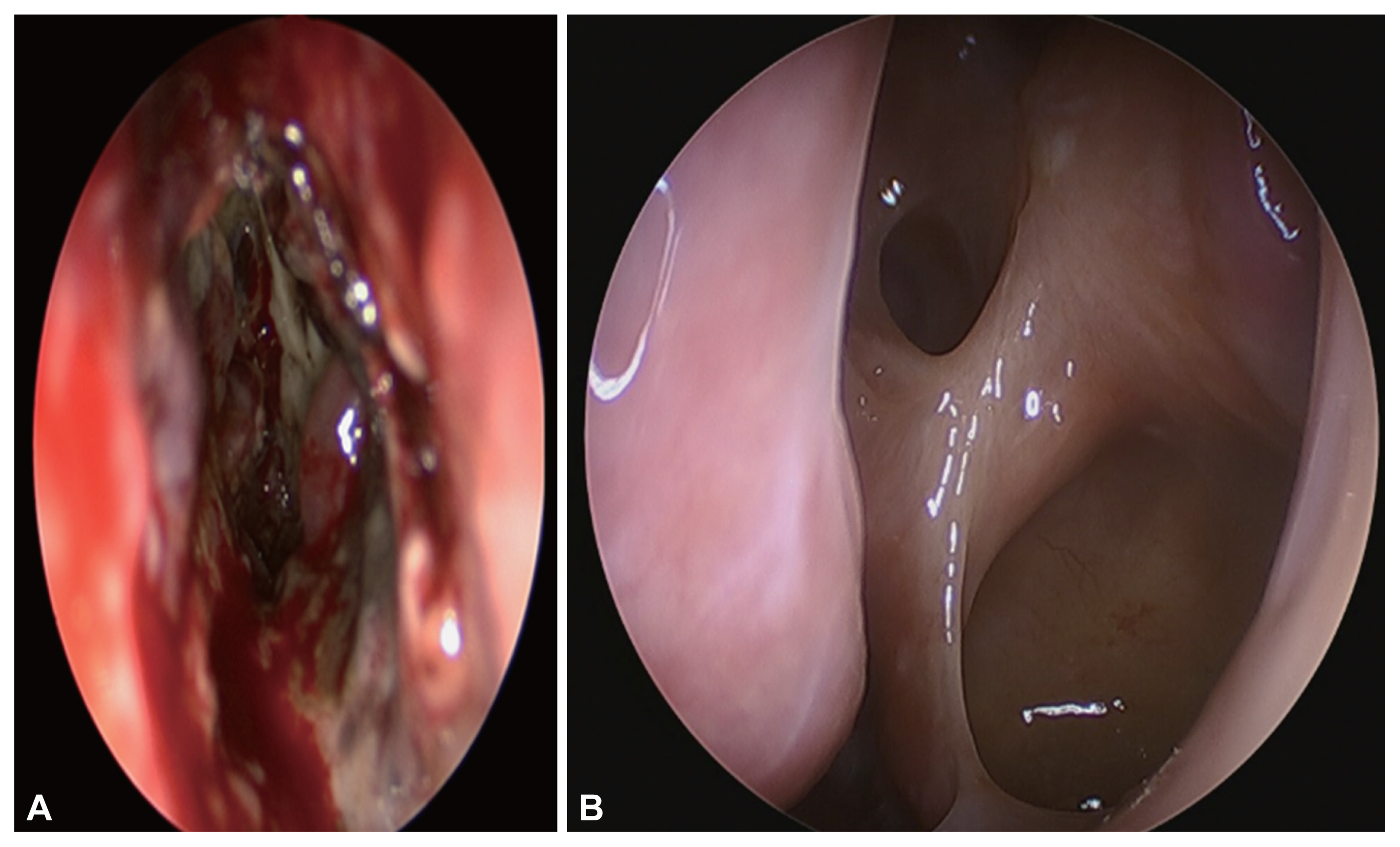
Fig. 5
Post-operative patient’s paranasal computed tomography view. A: Coronal view. B: Axial view. There is no lesion and no recurrence (indicated by stars). Nine months after treatment, the rim-enhanced lesion in left aspect of genu of the corpus callosum (indicated by arrow) (C) and ethmoid sinus (indicated by star) (D) was decreased compare with pre-operative magnetic resonance imaging.
REFERENCES
1) Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) working group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011;17(12):1859–67.


2) Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel) 2018;4(3):90.



3) Jeong W, Keighley C, Chen S. The epidemiology, management and outcomes of invasive mucormycosis in the 21st century: a systematic review. In: Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases; 2017 Apr 22–25; Vienna, Austria ECCMID. 2017;pp 1445.
4) Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg 2002;127(1):22–31.



5) Thurtell MJ, Chiu AL, Goold LA, Akdal G, Crompton JL, Ahmed R, et al. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Exp Ophthalmol 2013;41(6):567–76.


6) Anaissie EJ, Mattiuzzi GN, Miller CB, Noskin GA, Gurwith MJ, Mamelok RD, et al. Treatment of invasive fungal infections in renally impaired patients with amphotericin B colloidal dispersion. Antimicrob Agents Chemother 1998;42(3):606–11.




7) Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis 2012;54(Suppl 1):S73–8.



8) DiBartolo MA, Kelley PS. Rhino-orbital-cerebral mucormycosis (ROCM): a comprehensive case review. Aviat Space Environ Med 2011;82(9):913–6.

-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,671 View
- 55 Download
- Related articles
-
Two Cases of Rhinocerebral Mucormycosis in Diabetic Patients2000 May;7(1)
A Case of Rhino-Orbito-Cerebral Mucormycosis2003 November;10(1, 2)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print