|
 |
| J Rhinol > Volume 30(1); 2023 |
|
Abstract
Background and Objectives
Epistaxis is one of the most common emergencies in otolaryngology, and the recently developed Rapid Rhino nasal pack, a balloon-type nasal packing device, is widely used in emergency departments. Rebleeding after initial treatment increases patients’ discomfort and medical costs. The aim of this study was to investigate risk factors for rebleeding in patients treated with Rapid Rhino packing.
Methods
In this retrospective study, 93 patients with epistaxis treated with Rapid Rhino from January 2020 to November 2022 were divided into the well-controlled group (39 patients) and the rebleeding group (54 patients), and the baseline characteristics, management methods, and complications were compared between these groups. The rebleeding group was divided according to whether patients experienced a single episode of rebleeding (38 patients) or multiple rebleeding episodes (16 patients), and the differences between these two groups were compared.
Results
Oral anticoagulation therapy was associated with a higher risk of rebleeding after Rapid Rhino packing (odds ratio [OR]=8.41, p=0.047). A history of nasal surgery was associated with multiple rebleeding (OR=22.55, p=0.009). Age, sex, the management method, complications, and the site of bleeding were not found to be related to rebleeding.
Conclusion
Patients with rebleeding after Rapid Rhino nasal packing had a higher rate of concurrent oral anticoagulation therapy. A history of nasal surgery was strongly associated with multiple episodes of rebleeding. A detailed medical history can be important for assessing the risk of rebleeding in epistaxis patients treated with Rapid Rhino packing.
Epistaxis is one of the most common emergencies in otolaryngology, with approximately 60% of the population experiencing it at least once in their lifetime. In most cases, bleeding is controlled without special treatment. However, 5% to 10% of patients may require medical intervention [1,2]. There are various methods of controlling epistaxis, such as manual compression, nasal packing, electrocautery, and vascular embolization. An appropriate method should be selected according to the patient’s condition [3].
Several temporary nasal packing devices have recently been developed that can be conveniently used for patients with epistaxis in the emergency department. The Rapid Rhino (Smith & Nephew Inc., Austin, TX, USA; approved by the US Food and Drug Administration) is a nasal tamponade device that is inserted into the bleeding nasal cavity, inflated, and removed after 24–72 hours (Fig. 1). The Rapid Rhino device avoids intense pain and prevents the possibility of chondronecrosis due to excessive electrocauterization [4,5]. Although Rapid Rhino and similar devices are easy to use without special equipment, their disadvantage is the risk of rebleeding. In previous studies, Rapid Rhino showed a similar rebleeding risk to Merocel [6], but treatment failure was more common than for other treatment methods that directly target the bleeding vessel, such as electrocauterization or surgery [7]. Multiple visits to the hospital due to rebleeding are inconvenient for patients and increase the burden of medical expenses; therefore, it is important to identify and prevent factors that affect rebleeding. However, no previous study has been conducted on rebleeding after Rapid Rhino treatment, and knowledge on this issue is insufficient.
In this study, we aimed to identify risk factors associated with rebleeding in patients treated with Rapid Rhino.
The data of patients with epistaxis who visited the emergency department at our secondary-referral hospital from January 2020 to November 2022 were reviewed retrospectively. No pediatric epistaxis patients visited during this period. In this study, patients who underwent Rapid Rhino nasal packing in the emergency department and visited the otolaryngology outpatient clinic 24 to 72 hours later for packing removal were included. Patients with traumatic epistaxis and those lost to follow-up or with incomplete medical records were excluded. In addition, patients who were immediately referred to the otolaryngology department because bleeding continued even after Rapid Rhino insertion in the emergency department were excluded. The clinical features of these patients are presented in the Supplementary Table 1 (in the online-only Data Supplement). The included patients were divided into two groups according to hemostasis results: the well-controlled group included those without further bleeding after Rapid Rhino packing removal at the outpatient clinic, while the rebleeding group included cases in which additional treatment was required due to continued bleeding after packing removal or delayed bleeding occurred within 1 week after removal. Within the rebleeding group, patients were further divided according to whether they visited the outpatient clinic twice or more within 1 month due to multiple bleeding events (the multiple rebleeding group) or they did not visit again after the first rebleeding event (the single rebleeding group). A flowchart of the study is shown in Fig. 1. This study was approved by the Institutional Review Board of Bundang Jesaeng General Hospital (approval number 2022-07-002). The requirement for informed consent was waived due to the retrospective nature of the study.
Rapid Rhino products vary from 45 mm to 90 mm in length. Since our hospital uses a single size of 75 mm for adult patients, all patients included in this study received a 75-mm Rapid Rhino. The surface of the device is coated with a lubricant and carboxymethylcellulose, which induces platelet aggregation. When air is injected through an attached pressure cuff, the device gently compresses the nasal mucosa to stop bleeding [8].
In this study, Rapid Rhino treatment was performed as follows. First, after sufficiently soaking the product in sterilized water, it was inserted parallel to the nasal floor. When the device was properly inserted into the desired position, it was inflated by injecting 4 mL to 5 mL of air into the pressure cuff. The amount of air was controlled by manipulating the pressure in the cuff (Fig. 2). All patients were observed for 30 minutes and returned home only when no further bleeding was confirmed. If persistent bleeding was observed, the patient was immediately referred to an otolaryngologist. Patients who returned home after successful initial treatment were asked to visit an otolaryngology outpatient clinic 24 to 72 hours later for nasal packing removal (Fig. 1).
The patients’ demographic and clinical data, including age, sex, comorbidities, history of epistaxis and nasal surgery, anticoagulant or antiplatelet use, and blood pressure measured at the emergency department visit were collected. Treatment data, including the time to removal of nasal packing, number of bleeding episodes, time to event of rebleeding, treatment method of the second bleeding event, adverse events, and bleeding focus, were also collected and analyzed.
Data are presented as mean values±standard deviation. All statistical analyses were performed using SPSS version 24 (IBM Corp., Armonk, NY, USA). The chi-square test, Fisher exact test, and independent t-test were used for comparisons between two groups. Odds ratios (ORs) were obtained with 95% confidence intervals (CIs) using logistic regression analysis. A two-sided p-value <0.05 was considered statistically significant.
Rebleeding occurred in 54 of 93 patients. The characteristics of the well-controlled group and the rebleeding group are shown in Table 1. There were no significant differences between the two groups in sex, age, location of bleeding, blood pressure, time to packing removal, comorbidities, and history of epistaxis and nasal surgery. The number of patients taking oral anticoagulants was 1 (2.6%) in the well-controlled group and 10 (18.5%) in the rebleeding group (p=0.022). The adjusted OR was 8.41 (95% CI, 1.03–68.78; p=0.047), meaning that patients in the rebleeding group were 8.41 times more likely to take oral anticoagulants than patients in the well-controlled group (Table 2). There was no significant difference between two groups in the use of antiplatelets.
The rebleeding group was divided into the multiple rebleeding group and the single rebleeding group according to the number of bleeding episodes, and the characteristics of the two groups are shown in Table 3. There were no significant differences in sex, age, location of bleeding, blood pressure in the emergency department, or comorbidities between the two groups, and no difference in the history of taking anticoagulants and antiplatelet drugs. In the multiple rebleeding group, five patients (31.3%) had undergone nasal surgery in the past, and in the single rebleeding group, one patient (2.6%) had a history of nasal surgery (p=0.007). The adjusted OR was 22.55 (95% CI, 2.17–234.35; p=0.009) (Table 4). Of the five patients who had nasal surgery in the multiple rebleeding group, three underwent septoturbinoplasty, one underwent septoturbinoplasty with endoscopic sinus surgery, and one patient did not remember the name of the surgical procedure. The elapsed time after surgery ranged from 4 years to 11 years. In the single rebleeding group, one patient underwent septoturbinoplasty two months prior to bleeding.
Forty-one patients (75.9%) in the rebleeding group underwent electrocautery at the first rebleeding. The bleeding focus in 36 patients (66.7%) in the rebleeding group was the anterior nasal septum. There were no significant differences between the two groups in the treatment method or bleeding focus at the first rebleeding episode (Table 3).
Epistaxis is a relatively common condition and is self-limiting in most cases. However, rebleeding after initial treatment increases patients’ discomfort and the burden of medical expenses [9]. Among the newly developed convenient hemostatic products, Rapid Rhino is increasingly used in emergency departments, but the risk of rebleeding is relatively high due to blind insertion of the device [7]. The purpose of our study was to identify risk factors for rebleeding in patients treated with Rapid Rhino.
In this study, oral anticoagulation therapy was associated with an increased risk of rebleeding. However, the relationship between antiplatelet medication history and rebleeding was not significant. Anticoagulants are commonly prescribed to prevent thromboembolism in patients with cardiovascular disease, and previous studies have also identified anticoagulant use as a risk factor for rebleeding. It has been reported that patients taking anticoagulants have a high risk of recurrent epistaxis, longer hospital stays, and difficulties in hemostasis [10,11]. Consistent with the results of this study, Abrich et al. [11] reported that warfarin increased the risk of recurrent bleeding, while aspirin did not.
Other demographic factors, including age, sex, and comorbidities (e.g., hypertension) were not associated with rebleeding in this study. The relationship between high blood pressure and epistaxis has long been a matter of debate, and recent studies have suggested that high blood pressure increases the risk of recurrent epistaxis and massive bleeding [11-15]. The mechanism has been explained as an increase in nasal venous pressure due to atherosclerotic changes in patients with hypertension. In this study, neither systolic nor diastolic blood pressure was correlated with rebleeding, unlike previous studies. This discrepancy may have been due to the small number of subjects or a lack of accurate data, as the patients’ blood pressure was measured only once in the emergency department. Previous studies have not established a clear relationship between age and rebleeding. While Liao et al. [12] reported that the risk of intractable epistaxis increased with age, Abrich et al. [11] and Ando et al. [16] found no association between age and rebleeding. In our study, the proportion of male patients was higher in the multiple rebleeding group than in the other groups, but there was no statistically significant difference. This result might have been due to the small number of patients, but in addition to this, the relationship between sex and rebleeding has not been clearly established. While Kallenbach et al. [17] reported that men were readmitted for nosebleeds more frequently than women, Abrich et al. [11] found no significant sex difference in the recurrence of bleeding.
In this study, a history of nasal surgery was more frequent in the multiple rebleeding group than in the single rebleeding group. Most cases of postoperative bleeding after sinonasal surgery occur within a few days, and delayed bleeding is known to occur within an average of 3 weeks [18]. Surgery itself may not be a direct cause of bleeding, given that patients in this study underwent surgery at least 2 months and up to 11 years prior to bleeding. However, a previous study by Seidel et al. [19] reported that the frequency of epistaxis was higher in patients with sinusitis or rhinitis, and in this study, four out of five patients with a history of nasal surgery in the multiple rebleeding group underwent septoturbinoplasty and/or endoscopic sinus surgery. Considering previous studies [20,21] showing that nasal discharge symptoms persisted in some patients even after septoturbinoplasty or endoscopic sinus surgery, an association between remaining sinonasal disease and rebleeding can be considered [19].
This study has several limitations. First, Rapid Rhino was inserted as an initial treatment, so the bleeding focus could not be evaluated. Therefore, we could not confirm the results of previous research reporting that the risk of rebleeding was higher in cases of posterior bleeding or unclear bleeding points than in cases of anterior bleeding [16,22]. Second, differences in cuff pressure applied to each patient may have affected hemostasis outcomes. Mackeith et al. [23] reported that the intranasal pressure varied among patients even when the same amount of air was injected. Conducting a follow-up study with the same cuff pressure, considering individual intranasal anatomical differences, would be helpful for evaluating the risk of rebleeding according to each patient’s characteristics. Third, the number of study subjects was relatively small (n=93), which may have been insufficient to detect statistically significant relationships for previously reported risk factors such as age, heart disease, and hypertension [11,22]. Large-scale studies are needed in the future. A prospective, randomized controlled study comparing the efficacy and safety of Rapid Rhino versus otolaryngological management would also be needed to clarify the clinical impact of this study.
The strength of this study is that it is the first study on rebleeding after treatment with Rapid Rhino, a temporary packing device that is increasingly used. A prospective study comparing Rapid Rhino packing with other hemostasis methods in patients taking anticoagulants and a history of nose surgery, which were shown to be significant risk factors for rebleeding in this study, would be meaningful.
In conclusion, patients with rebleeding after Rapid Rhino nasal packing had a higher rate of concurrent oral anticoagulation therapy. A history of nasal surgery was also strongly associated with multiple episodes of rebleeding. A detailed medical history can be important for assessing the risk of rebleeding in epistaxis patients treated with a Rapid Rhino.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.18787/jr.2023.00001.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Mi Rye Bae. Data curation: Joon Taek Oh. Formal analysis: Mi Rye Bae, Joon Taek Oh. Methodology: Mi Rye Bae. Supervision: Mi Rye Bae. Validation: Mi Rye Bae. Visualization: Joon Taek Oh. Writing—original draft: Joon Taek Oh. Writing—review & editing: Mi Rye Bae.
Fig. 1.
A schematic diagram of the management flow of epistaxis patients and participant selection process in this study.
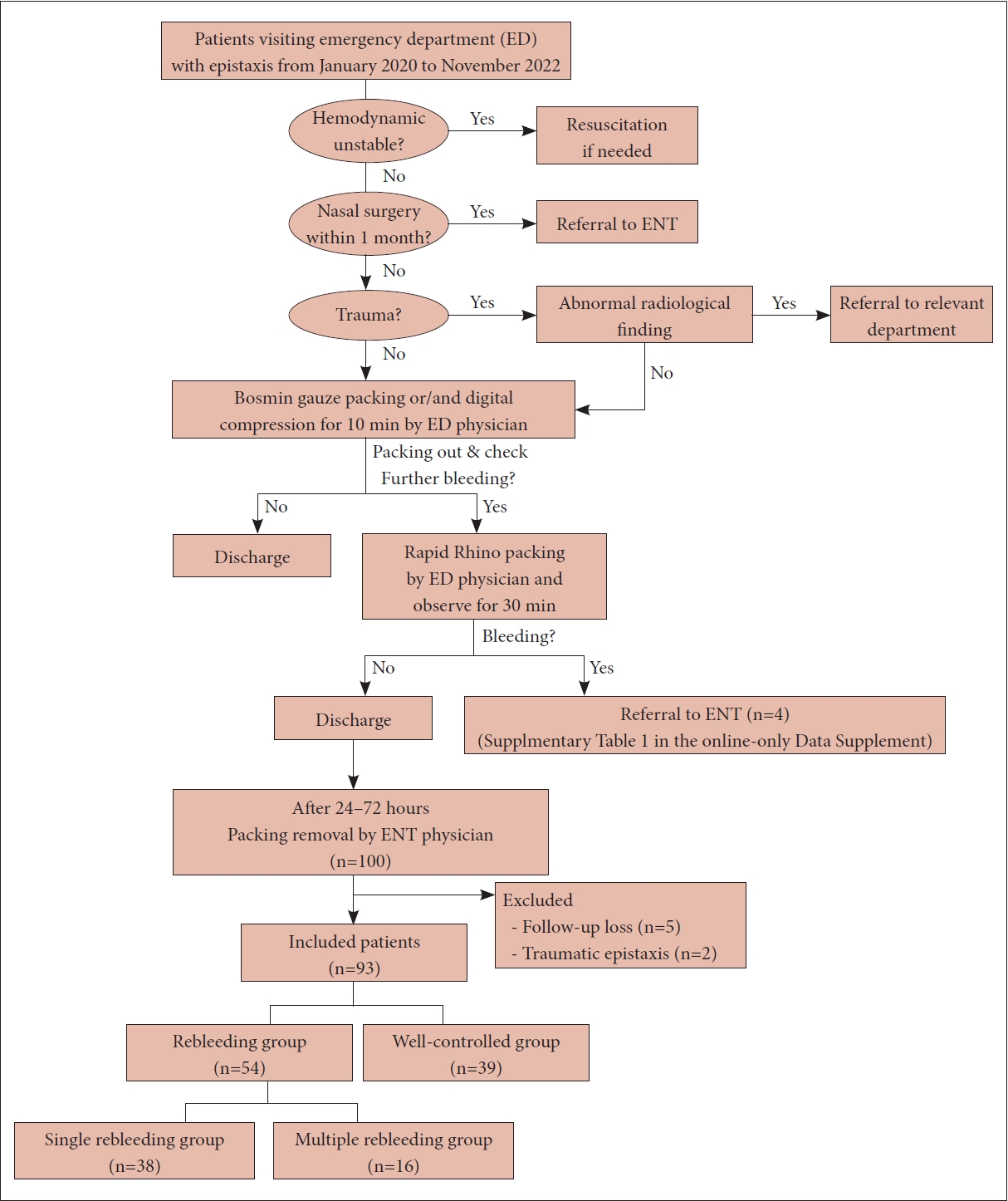
Fig. 2.
Utilization of a Rapid Rhino nasal pack. A: Soak the device in sterile water for 30 seconds. B: Insert along the nasal floor. C: Inflate the balloon with air. D: Tape to the patient’s cheek. Remove after 24–72 hours.
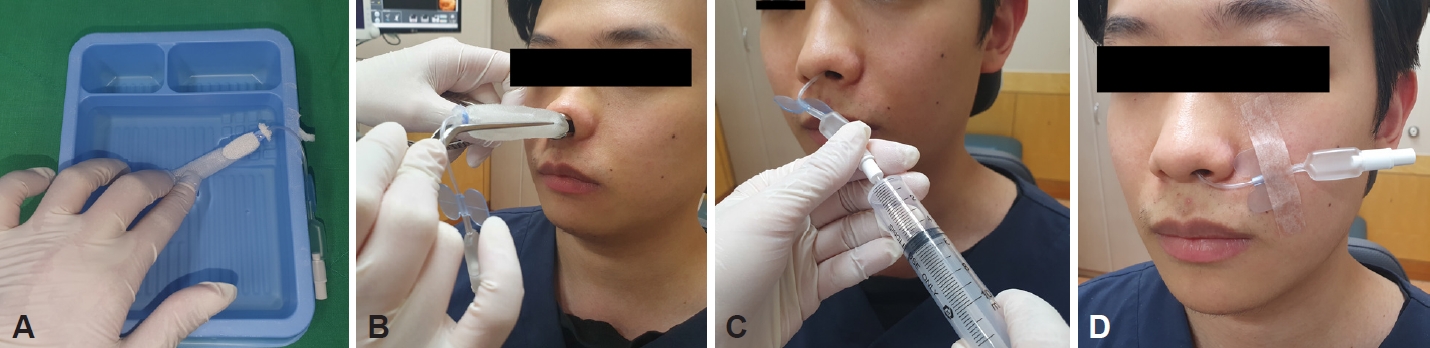
Table 1.
Baseline characteristics and outcomes of the study groups
| Variables | Total (n=93) |
Rebleeding |
|||
|---|---|---|---|---|---|
| Well controlled (n=39) | Rebleeding (n=54) | p-value | |||
| Age (yr) | 61.3±18.5 | 59.7±19.9 | 62.4±17.4 | 0.489 | |
| Sex | 0.564 | ||||
| Male | 65 (69.8) | 26 (66.7) | 39 (72.3) | ||
| Female | 28 (30.2) | 13 (33.3) | 15 (27.7) | ||
| History of epistaxis | 15 (16.1) | 3 (7.6) | 12(22.2) | 0.086* | |
| History of nasal surgery | 8 (8.6) | 2 (5.1) | 6 (11.1) | 0.461* | |
| Rhinoplasty | 1 | 1 | 0 | ||
| Septorhinoplasty | 1 | 1 | 0 | ||
| Septoturbinoplasty | 4 | 0 | 4 | ||
| Endoscopic sinus surgery and septoturbinoplasty | 1 | 0 | 1 | ||
| Unknown | 1 | 0 | 1 | ||
| Location | 0.796 | ||||
| Right | 51 (54.8) | 22 (56.4) | 29 (53.7) | ||
| Left | 42 (45.2) | 17 (43.6) | 25 (46.3) | ||
| Elapsed time from packing to removal (hr) | 40.4±18.1 | 41.6±17.4 | 39.5±18.7 | 0.589 | |
| SBP in ED (mm Hg) | 155.4±22.9 | 153.6±21.9 | 156.6±23.8 | 0.534 | |
| DBP in ED (mm Hg) | 94.6±18.4 | 94.8±18.6 | 94.4±18.4 | 0.904 | |
| Hypertension | 31 (33.3) | 14 (35.9) | 17 (31.5) | 0.656 | |
| Diabetes mellitus | 5 (5.4) | 1 (2.6) | 4 (7.4) | 0.395* | |
| Cardiovascular disease | 17 (18.3) | 5 (12.8) | 12 (22.2) | 0.247 | |
| Malignancy | 2 (2.2) | 0 (0) | 2 (3.7) | 0.508* | |
| Respiratory disease | 2 (2.2) | 1 (2.6) | 1 (1.9) | >0.999* | |
| Chronic liver disease | 2 (2.2) | 1 (2.6) | 1 (1.9) | >0.999* | |
| Anticoagulants | 11 (11.8) | 1 (2.6) | 10 (18.5) | 0.022* | |
| Antiplatelets | 21 (22.6) | 7 (17.9) | 14 (25.9) | 0.364 | |
| Complications | 7 | 2 (5.1) | 5 (9.3) | 0.695* | |
| Nasal septal perforation | 2 | 2 | 0 | ||
| Nasal synechia | 3 | 0 | 3 | ||
| Septal perforation and synechia | 1 | 0 | 1 | ||
| Admission for bleeding control under general anesthesia | 1 | 0 | 1 | ||
Table 2.
Odds ratios for rebleeding according to each factor compared to the well-controlled group
Table 3.
Comparison of baseline characteristics and outcomes between the multiple rebleeding group and the single rebleeding group
| Variable | Rebleeding group (n=54) |
Number of rebleeding episodes |
||||
|---|---|---|---|---|---|---|
| Single, 1 (n=38) | Multiple, ≥2 (n=16) | p-value | ||||
| Age (yr) | 62.4±17.4 | 63.3±18.2 | 60.4±15.8 | 0.394 | ||
| Sex | 0.508* | |||||
| Male | 39 (72.3) | 26 (68.4) | 13 (81.3) | |||
| Female | 15 (27.7) | 12 (31.6) | 3 (18.7) | |||
| History of epistaxis | 12 (22.2) | 11 (28.9) | 1 (6.3) | 0.084* | ||
| History of nasal surgery | 6 (11.1) | 1 (2.6) | 5 (31.3) | 0.007* | ||
| Septoturbinoplasty | 4 | 1 | 3 | |||
| Endoscopic sinus surgery and septoturbinoplasty | 1 | 0 | 1 | |||
| Unknown | 1 | 0 | 1 | |||
| Location | 0.772* | |||||
| Right | 29 (53.7) | 21 (55.3) | 8 (50.0) | |||
| Left | 25 (46.3) | 17 (44.7) | 8 (50.0) | |||
| Elapsed time from packing to removal (hr) | 39.5±18.7 | 40.9±20.0 | 36.1±15.2 | 0.629 | ||
| SBP in ED (mm Hg) | 156.6±23.8 | 153.9±24.7 | 163.1±20.4 | 0.116 | ||
| DBP in ED (mm Hg) | 94.4±18.4 | 92.0±17.5 | 99.9±20.0 | 0.247 | ||
| Hypertension | 17 (31.5) | 11 (28.9) | 6 (37.5) | 0.540* | ||
| Diabetes mellitus | 4 (7.4) | 3 (7.9) | 1 (6.3) | >0.999* | ||
| Cardiovascular disease | 12 (22.2) | 10 (26.3) | 2 (12.5) | 0.474* | ||
| Malignancy | 2 (3.7) | 1 (2.6) | 1 (6.3) | 0.509* | ||
| Respiratory disease | 1 (1.9) | 1 (2.6) | 0 (0) | >0.999* | ||
| Chronic liver disease | 1 (1.9) | 0 (0) | 1 (6.3) | 0.296* | ||
| Anticoagulants | 10 (18.5) | 7 (18.4) | 3 (18.8) | >0.999* | ||
| Antiplatelets | 14 (25.9) | 10 (26.3) | 4 (25) | >0.999* | ||
| Complications | 5 (9.3) | 2 (5.2) | 3 (18.8) | 0.148* | ||
| Septal perforation | 0 | 0 | 0 | |||
| Synechia | 3 | 2 | 1 | |||
| Septal perforation and synechia | 1 | 0 | 1 | |||
| Admission for bleeding control under general anesthesia | 1 | 0 | 1 | |||
| Treatment of first rebleeding | ||||||
| Electrocauterization | 41 (75.9) | 31 (81.6) | 10 (62.5) | 0.134 | ||
| Nasal packing | 9 (16.7) | 5 (13.2) | 4 (25) | 0.425* | ||
| Rapid rhino | 4 | 4 | 0 | |||
| Merocel | 2 | 0 | 2 | |||
| Surgicel | 3 | 1 | 2 | |||
| Chemocauterization with Albothyl (policresulen solution) | 2 (3.7) | 1 (2.6) | 1 (6.3) | 0.509* | ||
| Observation | 2 (3.7) | 1 (2.6) | 1 (6.3) | 0.509* | ||
| Bleeding focus of rebleeding | ||||||
| Anterior septum | 36 (66.7) | 25 (65.8) | 11 (68.8) | 0.833 | ||
| Posterior septum | 4 (7.4) | 4 (10.5) | 0 (0) | 0.306* | ||
| Middle turbinate | 1 (1.9) | 2 (5.3) | 2 (12.5) | 0.573* | ||
| Inferior turbinate | 2 (3.7) | 2 (5.3) | 0 (0) | >0.999* | ||
| Middle meatus | 1 (1.9) | 0 (0) | 1 (6.3) | 0.296* | ||
| Inferior meatus | 5 (9.3) | 4 (10.5) | 1 (6.3) | >0.999* | ||
| Unknown | 2 (3.7) | 1 (2.6) | 1 (6.3) | 0.509* | ||
Table 4.
ORs for multiple rebleeding for each factor compared to the single rebleeding group
References
1) Buiret G, Pavic M, Pignat JC, Pasquet F. Gelatin-thrombin matrix: a new and simple way to manage recurrent epistaxis in hematology units. Case Rep Otolaryngol 2013;2013:851270.




2) Reyre A, Michel J, Santini L, Dessi P, Vidal V, Bartoli JM, et al. Epistaxis: the role of arterial embolization. Diagn Interv Imaging 2015;96(7-8):757–73.


3) Tunkel DE, Anne S, Payne SC, Ishman SL, Rosenfeld RM, Abramson PJ, et al. Clinical practice guideline: nosebleed (epistaxis). Otolaryngol Head Neck Surg 2020;162(1_suppl):S1–38.


4) Yang X, Yi K, Tian J, Guo Y. [A meta-analysis compare rapid rhino with Merocel for nasal packing]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012 26(14):655–60. Chinese.
5) Moumoulidis I, Draper MR, Patel H, Jani P, Price T. A prospective randomised controlled trial comparing Merocel and Rapid Rhino nasal tampons in the treatment of epistaxis. Eur Arch Otorhinolaryngol 2006;263(8):719–22.



6) Badran K, Malik TH, Belloso A, Timms MS. Randomized controlled trial comparing Merocel® and RapidRhino® packing in the management of anterior epistaxis. Clin Otolaryngol 2005;30(4):333–7.


7) Nikolaou G, Holzmann D, Soyka MB. Discomfort and costs in epistaxis treatment. Eur Arch Otorhinolaryngol 2013;270(8):2239–44.



8) Gudziol V, Mewes T, Mann WJ. Rapid Rhino: a new pneumatic nasal tamponade for posterior epistaxis. Otolaryngol Head Neck Surg 2005;132(1):152–5.



9) Pallin DJ, Chng YM, McKay MP, Emond JA, Pelletier AJ, Camargo CA Jr. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med 2005;46(1):77–81.


10) Denholm SW, Maynard CA, Watson HG. Warfarin and epistaxis--a case controlled study. J Laryngol Otol 1993;107(3):195–6.


11) Abrich V, Brozek A, Boyle TR, Chyou PH, Yale SH. Risk factors for recurrent spontaneous epistaxis. Mayo Clin Proc 2014;89(12):1636–43.


12) Liao Z, Guo J, Mi J, Liao W, Chen S, Huang Y, et al. Analysis of bleeding site to identify associated risk factors of intractable epistaxis. Ther Clin Risk Manag 2021;17:817–22.




14) Byun H, Chung JH, Lee SH, Ryu J, Kim C, Shin JH. Association of hypertension with the risk and severity of epistaxis. JAMA Otolaryngol Head Neck Surg 2020;147(1):1–7.



15) Min HJ, Kang H, Choi GJ, Kim KS. Association between hypertension and epistaxis: systematic review and meta-analysis. Otolaryngol Head Neck Surg 2017;157(6):921–7.



16) Ando Y, Iimura J, Arai S, Arai C, Komori M, Tsuyumu M, et al. Risk factors for recurrent epistaxis: importance of initial treatment. Auris Nasus Larynx 2014;41(1):41–5.


17) Kallenbach M, Dittberner A, Boeger D, Buentzel J, Kaftan H, Hoffmann K, et al. Hospitalization for epistaxis: a population-based healthcare research study in Thuringia, Germany. Eur Arch Otorhinolaryngol 2020;277(6):1659–66.




18) Wang L, Wang X, Ba Y. Clinical analysis of delayed epistaxis following endoscopic sinus surgery. Am J Otolaryngol 2022;43(3):103406.


19) Seidel DU, Jacob L, Kostev K, Sesterhenn AM. Risk factors for epistaxis in patients followed in general practices in Germany. Rhinology 2017;55(4):312–8.


20) Schmelzer B, Katz S, Vidts G. Long-term efficacy of our surgical approach to turbinate hypertrophy. Am J Rhinol 1999;13(5):357–61.



21) Koskinen A, Myller J, Mattila P, Penttilä M, Silvola J, Alastalo I, et al. Long-term follow-up after ESS and balloon sinuplasty: comparison of symptom reduction and patient satisfaction. Acta Otolaryngol 2016;136(5):532–6.


- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print