The Relationship Between Zonulin and Asthma: A Mouse Model Study
Article information
Abstract
Background and Objectives
Zonulin is a human protein that regulates intercellular tight junctions and increases the permeability of the intestinal epithelium. In light of the increasing focus on zonulin’s role in numerous chronic inflammatory diseases, this study aimed to investigate whether differences exist in serum zonulin levels and bronchial epithelium zonulin expression in vivo between asthma and normal groups, using a mouse model.
Methods
Sixteen mice were utilized in this study, divided evenly between the normal and asthma groups. Serum zonulin levels, the expression of zonulin antibody in the bronchial epithelium, and serum cytokine levels were evaluated in both groups. Enzyme-linked immunosorbent assay and RNA in situ hybridization were utilized for the analysis.
Results
The asthma group exhibited significantly higher levels of serum zonulin. High zonulin antibody expression was also observed in the bronchial epithelium of the asthma group. Given that our mouse model demonstrated a significant difference in interleukin (IL)-4 and IL-6 between the normal and asthma groups, zonulin may be associated not only with type 2 responses but also with various subtypes of asthma. Further studies are required to investigate this relationship in greater detail.
Conclusion
Zonulin may play a role in the complex pathophysiology of asthma and could serve as a biomarker in various asthma-related situations.
INTRODUCTION
Zonulin is a protein homologous to the zonula occludens toxin found in Vibrio cholerae. It is known to increase the permeability of the intestinal epithelium by affecting intercellular tight junctions [1]. Since its initial discovery [1], numerous studies have investigated the role of zonulin in various diseases, including autoimmune diseases, neurodegenerative disorders, and neoplasms [2]. In recent years, increasing attention has also been paid to the role of zonulin in multiple chronic inflammatory diseases [3].
Increased intestinal permeability has also been reported in asthma [4,5]. It has been proposed that asthma might influence the entire human mucosal immune system [4]. Furthermore, recent pioneering studies have suggested that serum zonulin levels could serve as a biomarker for asthma severity [6,7]. However, it is important to note that these studies have some limitations, such as the still-unclear causal relationship between serum zonulin levels and asthma severity [6] and limited control of confounding factors [7]. Despite these limitations, zonulin should still be considered as a potential biomarker for asthma.
In this study, we compared serum zonulin levels, zonulin antibody expression in the bronchial epithelium, as well as various cytokine levels, between normal and asthmatic mice. Based on a comprehensive review of the existing literature, we propose a potential role for zonulin measurements in the context of asthma.
METHODS
Materials
Lipopolysaccharide (LPS; Escherichia coli O55:B5) and a mouse TH17 magnetic bead panel (MTH17MAG-47K) were purchased from MilliporeSigma (Burlington, MA, USA). Anti-haptoglobin antibody was obtained from Abcam (Cambridge, UK) and phosphate-buffered saline (PBS) was purchased from BioNeer (Daejeon, Republic of Korea). A zonulin enzyme-linked immunosorbent assay (ELISA) kit was obtained from Immundiagnostik (Bensheim, Germany).
Asthma mouse model preparation
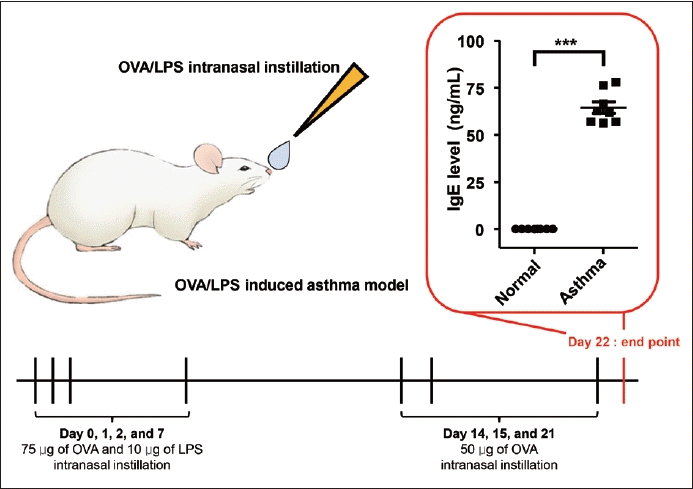
All animal experiments were performed under the guidelines of the Institutional Animal Care and Use Committee of KNOTUS (19-KE-420). c57bl6 male mice were purchased at 5 weeks of age from Orient Bio Inc. (Seoul, Republic of Korea). In this study, we utilized 16 mice and divided evenly them into normal and asthmatic groups. The asthma mouse model was induced through sensitization and challenges by ovalbumin (OVA) and LPS (Fig. 1) [8]. The OVA and LPS were prepared completely dissolved in PBS. The mice were first sensitized via intranasal instillation of the OVA/LPS solution (specifically, 75 μg/head OVA and 10 μg/head LPS) on days 0, 1, 2, and 7. Subsequently, the sensitized mice received intranasal instillations of OVA (50 μg/head) on days 14, 15, and 21. The control group was treated only with the PBS solution. Twenty-four hours after the final OVA instillation, we collected blood samples from the anesthetized mice via cardiac puncture, and then euthanized them using CO2 asphyxiation. The bronchial tissue from the mice was harvested and preserved in 10% phosphate-buffered formalin for future use. Blood samples were collected in a vacutainer tube containing a clot activator and then incubated at room temperature for 15 minutes. The serum was separated by centrifugation at 3,000 rpm for 10 minutes and reserved for subsequent experiments. We confirmed the establishment of the asthma disease model by measuring the immunoglobulin E (IgE) level using ELISA (Fig. 1).
Measurement of serum zonulin levels and zonulin expression in the bronchial tissue
The concentration of zonulin levels in the serum was determined by ELISA, following the manufacturer’s protocols provided by Immundiagnostik (Bensheim, Germany). RNA in situ hybridization (ISH) analysis for zonulin was conducted on paraffin-embedded bronchial tissues. To detect the target RNA in individual cells, we utilized the RNAscope® ProbeMm-Hp (Cat. 532711; MDxK, Seoul, Republic of Korea). The procedure was manually executed in accordance with the manufacturer’s instructions from MDxK (Seoul, Republic of Korea). In brief, 4-μm paraffin-embedded sections underwent heat treatment for 1 hour at 60°C, followed by deparaffinization in xylene twice for 5 minutes each time. Subsequently, the sections were dehydrated by 100% ethanol twice for 2 minutes each time. Each section was then incubated for 2 hours at 40°C in a HybEZ hybridization oven after treating with the zonulin RNA probe. The slides were thoroughly rinsed with wash buffer reagent. Finally, the sections were stained with hematoxylin and prepared for observation and analysis.
Measurement of serum cytokine levels
The levels of serum cytokines were measured using a Luminex multiplex assay kit (mouse TH17 magnetic bead panel), following the manufacturer’s protocols (MilliporeSigma; Burlington, MA, USA). The cytokines targeted in this assay included interleukin (IL)-1β, IL-4, IL-6, IL-10, IL-13, IL-25/IL-17E, IL-33, and tumor necrosis factor (TNF)-α.
Statistics
The Mann-Whitney U test was utilized to compare serum zonulin levels, cytokine levels, and antibody intensity in pneumocytes between the normal group and the asthma group. Statistical analysis was conducted using Windows SPSS version 24.0 (IBM Corp., Armonk, NY, USA), with a significance level set at 5%.
RESULTS
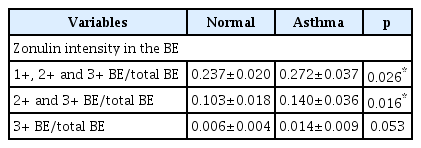
We investigated serum zonulin levels and zonulin expression in the bronchial epithelium. Zonulin expression was evaluated by single pathologist and the intensity of zonulin in each bronchial epithelial cell was categorized from 1+ to 3+, according to the intensity of the stained color. Zonulin intensity was scored by calculating the ratio of the area covered by each intensity category for the bronchial epithelium relative to the total bronchial epithelium. The score was compared between the normal and asthma groups. Additionally, we assessed various serum cytokine levels in both the normal and asthma groups, including IL-1β, IL-4, IL-6, IL-10, IL-13, IL-25/IL-17E, IL-33, and TNF-α. These cytokine levels were utilized to validate the characteristics of our asthma model. A summarized overview of the data is provided in Tables 1 and 2.
Serum zonulin levels and zonulin antibody expression in the bronchial epithelium
The mean serum zonulin level in the asthma group (65.88±21.86 ng/mL) was found to be significantly higher than that in the normal group (47.81±6.55 ng/mL, p=0.042) (Fig. 2). Zonulin expression was prominent in the bronchial epithelium of the asthma group (Fig. 3). Zonulin expression was undetectable in normal controls.
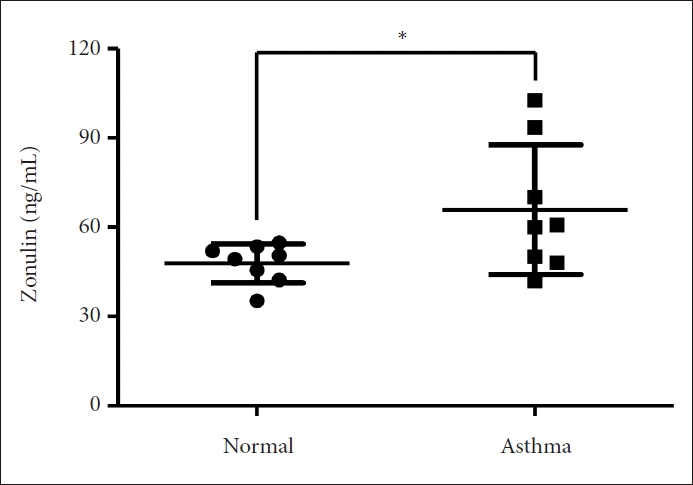
Serum zonulin levels in the normal and asthma groups. A bar graph shows the significant difference (p=0.042) in serum zonulin levels, measured using ELISA, in normal and asthmatic mouse. An asterisk (*p<0.05) in the graph represents significant difference. ELISA, enzyme-linked immunosorbent assay.

Zonulin antibody expression in the bronchial epithelium of asthmatic mice. A: Zonulin antibody expression in the bronchial epithelium in our asthma model. The zonulin antibody is marked by brown color (black arrows) in the bronchial epithelium (red circle). The RNA ISH technique was utilized for zonulin antibody identification. B: Representative image of zonulin antibody analysis using the RNA ISH technique. Antibody expression in the bronchial epithelium is highlighted in a red circle. This images were taken under 400× magnification. ISH, in situ hybridization.
Serum cytokine level
The mean serum levels of cytokines (IL-1β, IL-4, IL-6, IL-10, IL-13, IL-25/IL-17E, IL-33, and TNF-α) in the normal group were found to be 1.67±1.53, 0.82±0.15, 5.20±4.31, 4.65±0.84, 71.29±7.20, 964.12±156.38, 13.31±11.71, and 3.26±1.21 pg/mL, respectively. In the asthma group, the mean serum levels of cytokines were 10.39±8.28, 1.72±0.44, 36.61±17.17, 5.91±3.10, 93.99±19.38, 1,200.14±503.71, 21.2±6.70, and 4.61±4.10 pg/mL, respectively. Notably, significant differences were observed in IL-4 (p=0.019) and IL-6 (p=0.038) between the normal and asthma groups (Fig. 4).
DISCUSSION
Asthma is a prevalent chronic airway disease characterized by symptoms such as shortness of breath, chest tightness, and cough [9]. Impaired intercellular tight junctions and damaged epithelium in the airways are thought to contribute to the development or exacerbation of asthma, as they facilitate the penetration of environmental triggers into the epithelial layer [10]. This increased penetration has been suggested to worsen airway remodeling, leading to persistent chronic airway inflammation [11]. Consequently, intestinal penetration has been hypothesized to play a role in various allergic [12,13]. Recently, efforts have been made to analyze the relationship between zonulin, a surrogate marker for epithelial permeability, and asthma [6,7]. However, our understanding of the role of zonulin in the pathophysiology of asthma remains limited. Zonulin, a protein analogue of the zonula occludens toxin, binds to the proteinase-activating receptor of the epithelium, weakening the intercellular tight junction [2]. Currently, there are hypotheses suggesting that simultaneous mucosal changes occur in the intestinal mucosa following antigen exposure to the bronchial epithelium in asthma patients, but these require further investigation [6].
In this study, we observed that the overall serum zonulin levels were significantly higher in the asthma group than in the normal group. This observation is consistent with research conducted by Baioumy et al. [6], who studied house dust mite allergic asthma in humans. They discovered a significant correlation between serum zonulin levels, patient residence (urban vs. rural), and the severity of asthma. However, they did not observe a significant relationship with total serum IgE levels. Furthermore, another prospective human study reported a positive correlation between serum zonulin levels, bronchial epithelial zonulin expression, and the severity of asthma. However, they found no significant associations with total serum IgE levels or blood eosinophil counts [7].
It is important to note that both studies highlighted the challenges in adjusting for multiple confounding factors, including environmental and various allergen-related factors. Consequently, it would be difficult to conclude that the currently observed serum zonulin levels were solely due to asthma. Further research is needed to explore these relationships while carefully considering and controlling for potential confounders.
In our study, we observed a difference in zonulin antibody expression between the normal and asthma groups in the bronchial epithelium from the lung tissues. It has been proposed that increased intestinal permeability is present in allergic diseases including bronchial asthma [12], and zonulin antibody staining has been conducted in the intestinal epithelium of patients with various disorders [14-16]. However, studies on zonulin antibody expression in the bronchial epithelium have been less extensive than those on the intestinal epithelium, and the relationship between zonulin and airway inflammation still requires further evaluation. We believe that our current findings of increased zonulin antibody expression in asthmatic lung tissues will contribute to the ongoing research on zonulin in the field of airway inflammation.
We observed a significant difference in serum levels of IL-4 and IL-6 between the normal and asthma groups. IL-4 is a well-known cytokine associated with T helper 2 (Th2) cell-mediated diseases. It plays a role in B-cell class switching and IgE synthesis [9,17]. The elevated serum IL-4 levels in our asthma group suggest that our OVA-immunized asthma model mouse effectively represents a Th2-mediated reaction.
However, there was also a significant difference in serum IL-6 levels between the normal and asthma groups. Previous studies on asthma cohorts have shown that systemic inflammatory conditions such as obesity and older age are associated with severe asthma [18,19]. IL-6, which is primarily produced by adipose tissue cells, has emerged as a significant biomarker for understanding the factors contributing to severe asthma [20]. In fact, clinical trials are currently exploring the use of IL-6 inhibitors in treating asthma, suggesting that type 2 inflammation may not be the only therapeutic target for this condition [9,21]. Given the significant increase in serum IL-6 levels observed in our asthma model, serum zonulin levels could potentially serve as a biomarker for not only type 2 inflammatory asthma but also various other asthma subtypes. Furthermore, it is possible that the use of LPS in sensitizing and challenging the mouse model could have elevated the serum IL-6 level. As suggested by previous studies, an elevated IL-6 level might be the result of inhibition of LPS-induced fibrinolysis in mice [22,23]. However, further research is required to elucidate this relationship.
The present study has several limitations. First, the sample size was small, which may limit the generalizability of the findings and the statistical power of the analysis. A larger sample size would provide more robust results and enhance the reliability of the conclusions. Second, confounding factors were not well controlled in this study. Confounding factors, such as individual heterogeneity of mice, could influence both the serum zonulin levels and cytokine outcomes. Failing to adequately account for these factors may introduce bias and limit the interpretation of the relationship between serum zonulin levels and cytokine levels. Future studies should aim to control for potential confounders more effectively to improve the validity of the findings. Third, since zonulin expression was undetectable in normal controls, we concluded that the asthma induced the zonulin expression in bronchial epithelium. However, future investigations with optimized techniques should be aimed to detect very small amounts of zonulin expression in normal controls, if possible, in order to quantitatively compare the amount of expression between the normal and asthma groups.
This study aimed to investigate the relationship between zonulin and asthma by evaluating serum zonulin levels and zonulin expression in the bronchial epithelium of a mouse model. Given the growing interest in the role of zonulin in the pathophysiology of asthma, it is crucial to continue conducting further research in Korea. By expanding the current knowledge and understanding of zonulin’s involvement in asthma, researchers can contribute to the development of new insights, diagnostic tools, and therapeutic strategies for this prevalent respiratory condition.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jeong-Hee Choi, Seok Jin Hong. Data curation: Sung Hun Kang, Jeong-Hee Choi. Formal analysis: Sung Hun Kang, Sun-Ju Byeon, Seok Jin Hong. Investigation: Joon-Pyo Hong, Hyunjoo Lee. Methodology: Joon-Pyo Hong, Sung Hun Kang, Sun-Ju Byeon. Supervision: Jinah Chu, Seok Jin Hong, Kyung Chul Lee. Validation: Jinah Chu, Sun-Ju Byeon, Seok Jin Hong. Visualization: Sung Hun Kang, Seok Jin Hong. Writing—original draft: Joon-Pyo Hong. Writing—review & editing: Jinah Chu, Seok Jin Hong.
Funding Statement
None