INTRODUCTION
Histamine has been recognized as a major mediator in allergic diseases such as allergic rhinitis, chronic rhinosinusitis, and nasal polyps [1]. Histamine gets released by lymphocytes, neutrophils, platelets, macrophages, and eosinophils, also endothelial cells and nasal lavage fluid produces histamine released factors [2,3]. This heterogeneous group of factors causing basophil degranulation may also recruit mast cell or basophil participation in late-phase allergic reactions and other chronic inflammatory conditions [4,5].
Histamine H1 receptor (H1R), originally derived from bovine, is known to be expressed in many human tissues including airway, intestinal and vascular smooth muscle and brain [6,7]. Histamine-1 receptor (H1R) antagonists control clinical symptoms related to histamine expression and H1R is a primary receptor of histamine activity [8]. Immunohistochemical studies have shown that strong immune responses to H1R in vascular endothelial cells increase vascular permeability, leukocyte infiltration, and edema formation [9]. Nevertheless, the mechanism of this effect has not been clearly understood.
Fibroblasts are a major structural component of tissue that provides mechanical strength by providing a fabric that supports the extracellular matrix (ECM) [10]. In the sinonasal mucosa, fibroblasts play a major role in a structural modification in myofibroblast differentiation affecting the pathogenesis of nasal polyps [11]. Fibroblasts are known to produce large amounts of the ECM such as collagen and fibronectin, and they also express many receptors for cytokines, growth factors, and hormones [9]. They are additionally considered to be responsible for local enrollment of inflammatory cells.
CXCL8 is considered to be a major source of neutrophil chemostimulants and induces changes and directional movement, exocytosis of stored proteins, and respiratory bursts [12]. CXCL8 is produced by inflammatory and non-inflammatory cells such as mononuclear cells, fibroblasts and epithelial cells [13]. Previous studies have shown a correlation between CXCL8 and allergic diseases such as asthma [14]. Histamine induces CXCL8 secretion in endothelial cells and alveolar macrophages [15]. These data suggest that histamine may also bring on a major role in CXCL8 production in nasal fibroblasts. Nevertheless, the mechanism by which histamine induces the expression of CXCL8 in nasal fibroblasts is uncertain. To understand the pathophysiology of sinonasal diseases, it is important to understand the mechanisms influencing the nasal patency.
The aim of this study is to determine the effect of histamine on CXCL8 production and histamine receptor activity and to investigate the mechanism underlying the effect in nasal fibroblasts.
MATERIALS AND METHODS
Chemicals and reagents
The reagents used in this experiment included histamine, diphenhydramine (first-generation H1R antagonist), pyrilamine (first-generation H1R antagonist), fexofenadine (second-generation H1R antagonist), and U-73122 (phospholipase C inhibitor). These five reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA). BAY11-7082 (NF-κB inhibitor) was purchased from Calbiochem (Billerica, MA, USA). Histamine, diphenhydramine, and pyrilamine were dissolved in distilled water. Fexofenadine, BAY11-7082, and pyrilamine were used in its dimethyl sulfoxide dissolved form. The final maximum concentration of dimethyl sulfoxide was below 0.1%.
Nasal tissues and nasal fibroblast culture
Eight normal control subjects were recruited for this experiment. All patients were recruited from the Department of Otolaryngology-Head and Neck Surgery, Korea University Medical Center. Only patients without allergies, asthma or aspirin sensitivity met inclusion criteria. Likewise, none of the participants experienced treatment with anti-allergic drugs for more than 2 months. Before obtaining nasal fibroblast cultures, informed consent was thoroughly explained and received from all participants. Normal control tissues from inferior turbinate specimens were gained by patients without any sinonasal diseases by rhinoplasty surgeries. The purity of cells was confirmed by its morphological characteristics such as spindle-shaped cell morphology and flow cytometry. Nasal fibroblasts were positive for vimentin, fibroblast markers, whereas negative for E-cadherin, epithelial cell marker. Once the cell confluency reached more than 80%, cells were enzymatically digested. The nasal fibroblasts were cultured for four passages [16]. The experiment was approved by the Korea University Medical Center Institutional Review Board.
Measurement of cell viability
Nasal fibroblasts were seeded in 96-well culture plates at a density of 104 cells/well, in culture medium and incubated for 24 hours. Fibroblasts were then treated with different concentrations of histamine or H1R antagonist for another 48 hours. Then nasal fibroblasts were incubated with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, Sigma) at a final concentration of 0.5 mg/mL. After 4 hours, MTT solution was discarded and 150 μL dimethyl sulfoxide (Sigma-Aldrich) was added to dissolve the formazan precipitate by shaking the plates for 10 minutes at mild speed on an orbital shaker. Microplate readers (F2000, Hitachi Ltd., Tokyo, Japan) were used to read the absorbance of each well at the wavelength of 540 nm.
Detection of histamine type 1 receptor mRNA in nasal fibroblasts
After nasal fibroblasts were stimulated by histamine (100 μM), total RNA was extracted by a Trizol reagent (Invitrogen, Carlsbad, CA, USA). The total amount of RNA was determined by an optical system using NanoDrop 2000 (Thermo Scientific, Wilmington, DE, USA). Real-time polymerase chain reaction was performed in Quantstudio3 (Applied Biosystems, Foster City, CA, USA) using Power SYBR Green PCR Master Mix (Applied Biosystems). Relative gene expression was calculated by evaluating real-time polymerase chain reaction data using the 2 (2DDCt) method. Experiments were repeated at least 3 times and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. A primer was added to perform the polymerase chain reaction; H1R (sense sequence 5’-GTC TAA CAC AGG CCT GGA TT-3’, antisense sequence 5’-GGA TGA AGG CTG CCA TGA TA-3’) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sense sequence, 5’-GTGGATATTGTCCCAT-3’ and antisense sequence, 5’-GCCCCAGCCTTCTTCATGGGG-3’).
CXCL8 measurements
The secretion levels of CXCL8 protein in nasal fibroblasts after treatment with histamine were evaluated by ELISA. Ten percentage of fetal bovine serum was used to incubate nasal fibroblasts and the supernatant media were collected. ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify CXCL8 expression according to manufacturer’s instructions. Ninety-six-well microplates were coated with diluted capture antibody and incubated overnight at room temperature. They were blocked with 1% bovine serum albumin for 1 hour and then added collected-supernatant media and standards for 2 hours. Detection antibody was added for 2 hours after washing. Streptavidin-horseradish peroxidase and its substrate were used for the manifestation of color. Optical density was set to 450 nm. The microtiter plate reader (SpectraMax 190, Molecular Devices, Sunnyvale, CA, USA) was used to determine the CXCL8 expression and each well was examined within 30 minutes.
Western blotting analysis
PRO-PREP TM protein extraction solution (iNtRON Biotechnology, Seongnam, Korea) was used to lyse nasal fibroblasts. The lysed materials were then placed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to polyvinylidene difluoride membranes (Millipore Inc., Billerica, MA, USA). Membranes were blocked for an hour in a 5% non-fat dry milk and were covered overnight in phospho (p)-p50, and total-p50 with 3% bovine serum albumin at 4ºC. Afterwards that, membranes were washed thoroughly for five minutes three times, and then treated with horseradish peroxide-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) for one hour. Then, substrates from an ECL system (Pierce, Rockford, IL, USA) were added for visualization.
Immunofluorescent staining
Nasal fibroblasts were seeded on coverslips and treated with histamine (100 μM). Nasal fibroblasts were fixed with 4% paraformaldehyde, and then permeabilized with 0.01% Triton X-100 in 1% bovine serum albumin for 10 minutes. After permeabilization, nasal fibroblast was blocked with 5% bovine serum albumin for 1 hour at room temperature. Nasal fibroblasts incubated with p-p50 (Sigma-Aldrich) overnight at 4ºC and then incubated with anti-rabbit Alexa 555 secondary antibodies (Invitrogen) for 1 hour. 4’-6-diamidino-2-phenylindole was applied for counterstaining of the nucleus. An LSM700 confocal laser scanning microscope (Zeiss, Oberkochen, Germany) was used for capturing and identifying stained cells.
Statistical data analysis
All statistical analyses were performed via SPSS Statistics 20.0.0 version (IBM Corp., Armonk, NY, USA). Statistically significant differences between groups were evaluated by one-way analysis of variation (ANOVA) for factorial comparisons and subsequently with Turkey’s post-hoc trial. Outcomes are given as mean±standard error of the men (SEM) which were extracted from at least three independent trials. Statistical significance was defined as p<0.05.
RESULTS
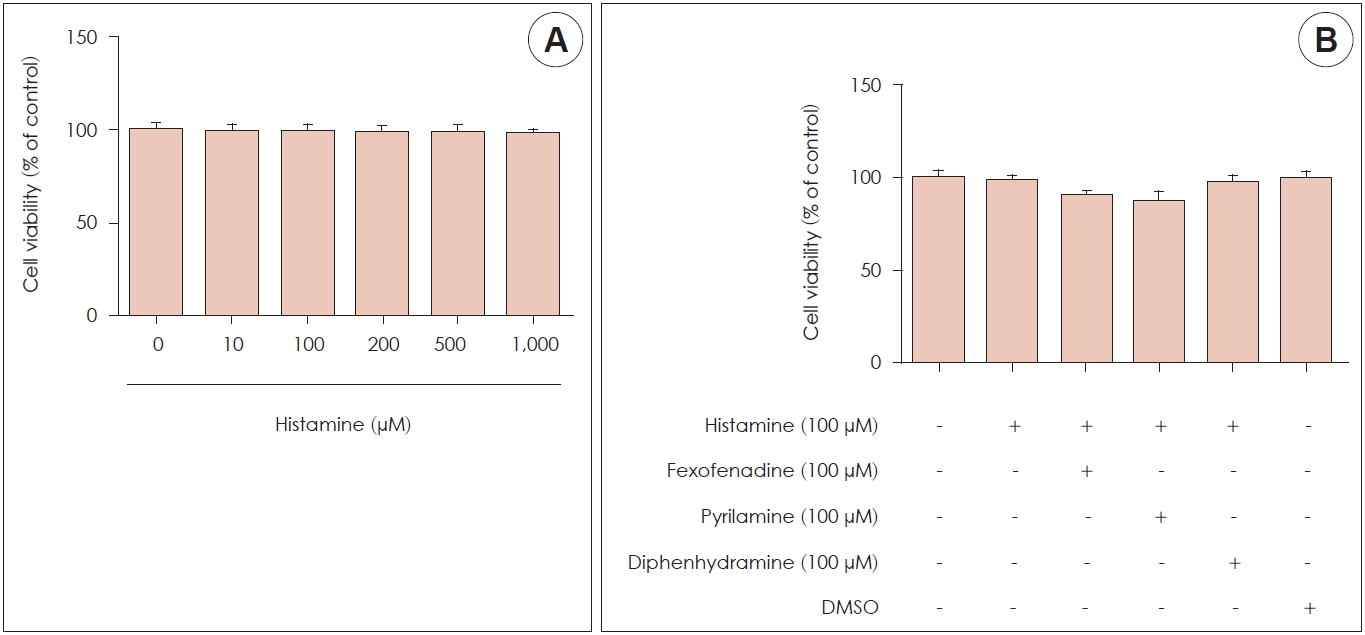
Effects of histamine on cytotoxicity
An MTT assay was used to examine the cytotoxicity of histamine and H1R antagonists. A cell titration curve was produced from serial dilutions of nasal fibroblasts and MTT reagent. The standard curve indicated a linear response between cell number and absorption at 570 nm. Nasal fibroblasts were examined at histamine concentrations ranging from 0 to 1,000 μM with various mixtures of histamine and H1R antagonist (fexofenadine, pyrilamine, and diphenhydramine). Cell survival was not affected by histamine until the concentration reached 1,000 μM (Fig. 1A), and it was also produced by different mixtures of histamine and each H1R antagonist for 72 hours (Fig. 1B).
Expression of H1R mRNA and effect of histamine on CXCL8 production
To investigate the CXCL8 expression in nasal fibroblasts, we measured CXCL8 expression using ELISA kit. H1R mRNA expression in nasal fibroblasts was identified by real-time polymerase chain reaction. H1R mRNA expression was increased significantly by histamine stimulation in a dose-dependent manner (Fig. 2A). ELISA was then used to examine whether histamine stimulated CXCL8 protein production in nasal fibroblasts. Nasal fibroblasts were treated with up to 200 μM histamine for 24 hours. CXCL8 protein expression increased significantly in response to histamine stimulation in a dose-dependent manner (Fig. 2B).
Effect of H1R antagonists on CXCL8 production of histamine-stimulated nasal fibroblasts
We investigated which histamine receptor was involved in the CXCL8 production. Nasal fibroblasts were pretreated with histamine receptor antagonists for H1R (fexofenadine, pyrilamine, diphenhydramine) and then stimulated by histamine for 24 hours. Fexofenadine, pyrilamine, and diphenhydramine significantly decreased CXCL8 production (Fig. 3A).
Effect of phospholipase C on histamine-induced CXCL8 production
To find the signaling pathway for histamine-induced CXCL8 production in nasal fibroblasts, stimulation of phospholipase C as a downstream marker of CXCL8 signaling was evaluated via ELISA. Histamine-induced CXCL8 protein production decreased in a dose-dependent manner after treatment with a phospholipase C inhibitor (U73122) (Fig. 3B). CXCL8 production was significantly decreased compared to the control after 10 μM treatments with a phospholipase C inhibitor.
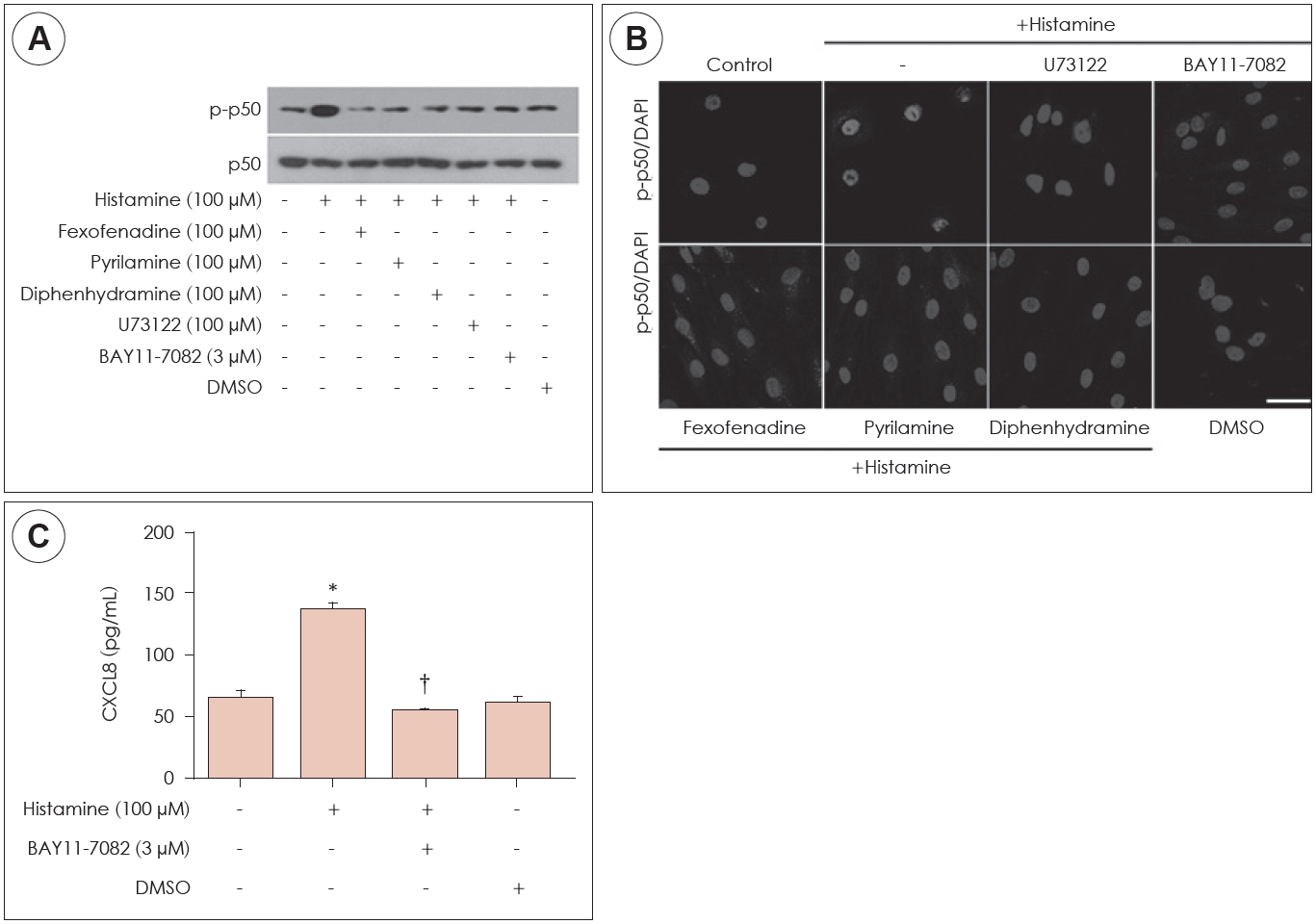
Effect of histamine on NF-κB activation in histamine-induced CXCL8 production
Next, the effect of NF-κB activation on CXCL8 production was confirmed in histamine-stimulated nasal fibroblasts. The expression levels of p-p50 were determined by western blot in nasal fibroblasts, in the absence or presence of H1R antagonist or BAY11-7082. Histamine increased expression of p-p50 and BAY11-7082 (NF-κB inhibitor) suppressed p-p50 expression (Fig. 4A). In immunocytochemical staining, histamine induced phosphorylation of p50 and translocation of p-p50 into the nucleus (Fig. 4B). The expression level of CXCL8 protein was determined by ELISA. Histamine induced expression of CXCL8 protein levels. However, BAY11-7082 inhibited expression of histamine-induced CXCL8 protein levels (Fig. 4C). These results indicated that histamine-induced CXCL8 production through NF-κB signaling pathway.
DISCUSSION
In the present study, we examined the effects of histamine on CXCL8 production via phospholipase C and NF-κB signaling pathways in nasal fibroblasts. The results showed a clear correlation between histamine signaling pathways and CXCL8 production, which was mediated primarily through H1R. H1R antagonists inhibited CXCL8 production. The phospholipase C inhibitor, U73122, significantly reduced the effect of histamine on CXCL8 production. The NF-κB inhibitor (BAY11-7082) inhibited CXCL8 protein production as effectively as fexofenadine in histamine-stimulated nasal fibroblasts. These effects supported the idea that H1R inhibited the activation of phospholipase C and NF-κB signaling pathways. Taken together, we suggest a signal pathway of histamine-induced CXCL8 through the H1R, phospholipase C and NF-κB signaling pathways.
Mast cells initiate immediate hypersensitivity reactions; after an IgE-dependent stimulation, mast cells rapidly release preformed mediators such as histamine [17]. Histamine plays a major role in the early clinical manifestations of allergic disorders. Mast cells reside in close proximity to potential target cells of mediator action such as fibroblasts, nerves, epithelial cells, and vascular endothelial cells. Histamine acts on target cells through distinct H1, H2, H3 and H4 receptors. Most of the effects of histamine in allergic disorders are mediated through HI and H2 receptors [7]. The production of CXCL8 is also augmented in human bronchial epithelial cells by histamine [18]. As in these human epithelial cells, histamine was found to stimulate the production of IL-6 and CXCL8 in human keratinocytes in the previous study [19]. Previous study reported that histamine stimulates the production of IL-6 and CXCL8, hence our results agreed to previous reports. As it was observed in the present study, we also found that the effect of histamine was blocked by an H1R antagonist in nasal fibroblasts.
CXCL8 (or IL-8) is an important chemokine that plays a crucial role in inflammatory pathways [13]. Monocytes/macrophages, epithelial cells, smooth muscle cells, and endothelial cells produce CXCL8 [19]. The main receptors that interact with CXCL8 have G-protein coupled seven transmembrane receptors, CXCR1 and CXCR2 [20]. A major effect of CXCL8 during the inflammatory process is chemotaxis of target cells to the site of inflammation, particularly neutrophils. CXCL8 also has chemotactic activity against T cells and basophils [18]. Neutrophil adhesion to and transmigration across the endothelium are regulated by CXCL8. Once neutrophils arrive at inflammation sites, CXCL8 further stimulates those cells to carry out phagocytosis, increasing the efficiency of tissue repair [21].
The role of cytokines in the development of nasal polyps and rhinosinusitis has been widely investigated. According to recent studies, increased levels of CXCL8 may participate in the primary pathogenesis of chronic rhinosinusitis, allergic rhinitis, and nasal polyps, as well as in recurrent episodes [22,23]. CXCL8 expression level in nasal polyps is higher than normal nasal tissue [24].
CXCL8 plays an important role in the inflammatory diseases, and its expression can be regulated through mechanisms. In previous studies, patients with cystic fibrosis showed higher levels of CXCL8 than patients without cystic fibrosis. The CXCL8 gene is regulated by NF-κB, AP-1, CAAT/enhancer-binding protein β (C/EBPβ or NF-IL-6), C/EBP homologous protein (CHOP), and cAMP response element binding protein (CREB). However, signaling pathways related to cytokine production have not been thoroughly evaluated in patients with nasal polyp and rhinosinusitis. Our previous study reported that PGE2 activated the Akt and NF-κB signaling pathways to produce IL-6 and CXCL8 in nasal fibroblasts [25].
In conclusion, these data add arguments to recent studies suggesting that histamine should be considered as an integral component of immune and inflammatory responses. We showed that CXCL8 production was stimulated by histamine via H1R, followed by downstream activation of phospholipase C and NF-κB signaling pathways. These results suggest that antihistamines are likely to affect multiple cytokines or chemokine in addition to blocking the more common effects of histamine in allergic inflammation. To investigate the clinical use of CXCL8 pathway production, further study into other pathways and in vivo applications are required.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print